Hi Ladies!
1stKris, please do not say anything to Tanya; or to my other kids either?
There are 3 of you on here that I trust implicitly. While I may open it up to a few select others, later, I want you three to know these things first...
This is for you WHEN YOU HAVE TIME.
It is very long- and I apologize for that- But I am "missing" something in figuring this all out.
It is sort of self-explanatory... I hope...
I have been swollen up like a mosquito that hit an artery ever since I had that "wrong" infusion. And I have also been feeling 'off'- I am not sure how to explain it to you.
I am also not sure what to do-
A different doctor? Yet, I know ALL of the damned doctors in this fucking pissant town are in cahoots.
I do believe I need a very good attorney. ?
Know any?
Read on-
More 'note' below
After you read "How to 'off' your patients without them knowing it." hehehe
* Pretty damn fine subtitle...__________________________________
http://www.drugs.com/cozaar.html
"Do not use potassium supplements or salt substitutes while you are taking Cozaar."
That's all I ever DO is salt subs- AND I also take potassium!!!
______________________________________________
______________________________________________
Then I went to drugs.com
Drug Interactions Results
Drug interactions for the following 13 drug(s):
Interactions between your selected drugs
potassium chloride ↔ losartan
Applies to: Klor-Con (potassium chloride), Cozaar (losartan)
MONITOR CLOSELY: Concomitant use of angiotensin II receptor blockers (ARBs) and potassium salts may increase the risk of hyperkalemia. Inhibition of angiotensin II results in decreased aldosterone secretion, which in turn causes potassium retention. Risk factors for developing severe or life-threatening hyperkalemia may include renal impairment, diabetes, old age, severe or worsening heart failure, dehydration, and concomitant use of other agents that block the renin-angiotensin-aldosterone system or otherwise increase serum potassium levels.
MANAGEMENT: Caution is advised if angiotensin II receptor blockers must be used concurrently with potassium salts, particularly in patients with renal impairment, diabetes, old age, severe or worsening heart failure, dehydration, or concomitant therapy with other agents that increase serum potassium such as nonsteroidal anti-inflammatory drugs, beta-blockers, cyclosporine, heparin, tacrolimus, trimethoprim, and licorice. The combination should generally be avoided in these patients unless absolutely necessary and the benefits outweigh the potential risks. Serum potassium and renal function should be checked prior to initiating therapy and regularly thereafter. Patients should be given counseling on the appropriate levels of potassium and fluid intake, and advised to seek medical attention if they experience signs and symptoms of hyperkalemia such as nausea, vomiting, weakness, listlessness, tingling of the extremities, paralysis, confusion, weak pulse, and a slow or irregular heartbeat.
methotrexate ↔ aspirin
Applies to: methotrexate, Ascomp with Codeine (aspirin/butalbital/caffeine/codeine)
GENERALLY AVOID: Salicylates may interfere with the renal elimination of methotrexate and may displace it from binding sites. The pharmacologic effect and toxicity of methotrexate may be increased. Patients receiving high-dose methotrexate are at a greater risk of developing toxicity.
MANAGEMENT: If these agents must be used concomitantly, caution should be exercised and the patient should be monitored closely for signs and symptoms of bone marrow suppression and nephrotoxicity. Patients should be advised to report possible symptoms of toxicity including nausea, vomiting, diarrhea, stomatitis, sore throat, chills, fever, rash, unusual bruising or bleeding, jaundice, dark urine, swelling of the extremities, or shortness of breath to their physician. Patients should also be counseled to avoid any other over-the-counter salicylate products.
ergotamine ↔ paroxetine
Applies to: Cafergot (caffeine/ergotamine), paroxetine
MONITOR CLOSELY: Concomitant use of agents with serotonergic activity such as serotonin reuptake inhibitors, monoamine oxidase inhibitors, tricyclic antidepressants, 5-HT1 receptor agonists, ergot alkaloids, lithium, St. John's wort, phenylpiperidine opioids, dextromethorphan, and tryptophan may potentiate the risk of serotonin syndrome, which is a rare but serious and potentially fatal condition thought to result from hyperstimulation of brainstem 5-HT1A and 2A receptors. Symptoms of the serotonin syndrome may include mental status changes such as irritability, altered consciousness, confusion, hallucinations, and coma; autonomic dysfunction such as tachycardia, hyperthermia, diaphoresis, shivering, blood pressure lability, and mydriasis; neuromuscular abnormalities such as hyperreflexia, myoclonus, tremor, rigidity, and ataxia; and gastrointestinal symptoms such as abdominal cramping, nausea, vomiting, and diarrhea.
MANAGEMENT: In general, the concomitant use of multiple serotonergic agents should be avoided if possible, or otherwise approached with caution if potential benefit is deemed to outweigh the risk. Patients should be closely monitored for symptoms of the serotonin syndrome during treatment. Particular caution is advised when increasing the dosages of these agents. The potential risk for serotonin syndrome should be considered even when administering serotonergic agents sequentially, as some agents may demonstrate a prolonged elimination half-life. For example, a 5-week washout period is recommended following use of fluoxetine before administering another serotonergic agent. If serotonin syndrome develops or is suspected during the course of therapy, all serotonergic agents should be discontinued immediately and supportive care rendered as necessary. Moderately ill patients may also benefit from the administration of a serotonin antagonist (e.g., cyproheptadine, chlorpromazine). Severe cases should be managed under consultation with a toxicologist and may require sedation, neuromuscular paralysis, intubation, and mechanical ventilation in addition to the other measures.
acetaminophen ↔ methotrexate
Applies to: acetaminophen/oxycodone, methotrexate
GENERALLY AVOID: Coadministration of methotrexate with other agents known to induce hepatotoxicity may potentiate the risk of liver injury. Methotrexate, especially at higher doses or with prolonged treatment, has been associated with hepatotoxicity including acute hepatitis, chronic fibrosis, necrosis, cirrhosis, and liver enzyme elevations.
MANAGEMENT: The use of methotrexate in combination with other potentially hepatotoxic agents (e.g., acetaminophen; alcohol; androgens and anabolic steroids; antituberculous agents; azole antifungal agents; ACE inhibitors; endothelin receptor antagonists; interferons; nucleoside reverse transcriptase inhibitors; retinoids; thiazolidinediones; anticonvulsants such as carbamazepine, hydantoins, felbamate, and valproic acid; lipid-lowering medications such as fenofibrate, HMG-CoA reductase inhibitors, and niacin; herbals and nutritional supplements such as black cohosh, chaparral, comfrey, DHEA, kava, pennyroyal oil, and red yeast rice) is generally not recommended unless the potential benefit outweighs the risk. Patients treated with methotrexate should be advised to seek medical attention if they experience potential signs and symptoms of hepatotoxicity such as fever, rash, itching, anorexia, nausea, vomiting, fatigue, right upper quadrant pain, dark urine, light-colored stools, and jaundice. Baseline and regular monitoring of hepatic function is recommended.
oxycodone ↔ butalbital
Applies to: acetaminophen/oxycodone, Ascomp with Codeine (aspirin/butalbital/caffeine/codeine)
MONITOR: Central nervous system- and/or respiratory-depressant effects may be additively or synergistically increased in patients taking multiple drugs that cause these effects, especially in elderly or debilitated patients.
MANAGEMENT: During concomitant use of these drugs, patients should be monitored for potentially excessive or prolonged CNS and respiratory depression. Ambulatory patients should be counseled to avoid hazardous activities requiring mental alertness and motor coordination until they know how these agents affect them, and to notify their physician if they experience excessive or prolonged CNS effects that interfere with their normal activities.
butalbital ↔ ergocalciferol
Applies to: Ascomp with Codeine (aspirin/butalbital/caffeine/codeine), Oysco 500 with D (calcium/vitamin d)
MONITOR: Coadministration with CYP450 inducers such as rifampin, isoniazid, barbiturates, and certain anticonvulsants may decrease the pharmacologic effects of vitamin D analogs. These agents are thought to induce the hepatic conversion of vitamin D to inactive metabolites and have been shown to reduce circulating levels of active vitamin D, sometimes accompanied by reduced serum calcium and increased parathyroid hormone levels. Patients on long-term anticonvulsant therapy have occasionally developed osteomalacia, presumably due to interference with vitamin D and calcium metabolism. There have also been isolated reports of patients who responded poorly to vitamin D supplements during treatment with phenytoin and/or primidone.
MANAGEMENT: Patients receiving vitamin D analogs with CYP450 inducers should be monitored for potentially reduced vitamin D effects. Dosage adjustments may be necessary.
codeine ↔ paroxetine
Applies to: |
Ascomp with Codeine (aspirin/butalbital/caffeine/codeine), paroxetine
MONITOR: Drugs that are inhibitors of CYP450 2D6 may interfere with the analgesic effect of codeine. The mechanism is decreased in vivo conversion of codeine to morphine, a metabolic reaction mediated by CYP450 2D6.
MANAGEMENT: The possibility of reduced or inadequate pain relief should be considered in patients receiving codeine with drugs that inhibit CYP450 2D6. An increase in the codeine dosage or a different analgesic agent may be necessary in patients requiring therapy with CYP450 2D6 inhibitors.
diazepam ↔ paroxetine
Applies to: diazepam, paroxetine
MONITOR: Central nervous system- and/or respiratory-depressant effects may be additively or synergistically increased in patients taking multiple drugs that cause these effects, especially in elderly or debilitated patients.
MANAGEMENT: During concomitant use of these drugs, patients should be monitored for potentially excessive or prolonged CNS and respiratory depression. Ambulatory patients should be counseled to avoid hazardous activities requiring mental alertness and motor coordination until they know how these agents affect them, and to notify their physician if they experience excessive or prolonged CNS effects that interfere with their normal activities.
aspirin ↔ paroxetine
Applies to: Ascomp with Codeine (aspirin/butalbital/caffeine/codeine), paroxetine
MONITOR: Serotonin reuptake inhibitors (SRIs) may potentiate the risk of bleeding in patients treated with ulcerogenic agents and agents that affect hemostasis such as anticoagulants, platelet inhibitors, thrombin inhibitors, thrombolytic agents, or agents that commonly cause thrombocytopenia. The tricyclic antidepressant, clomipramine, is also a strong SRI and may interact similarly. Serotonin release by platelets plays an important role in hemostasis, thus SRIs may alter platelet function and induce bleeding. Published case reports have documented the occurrence of bleeding episodes in patients treated with psychotropic agents that interfere with serotonin reuptake. Bleeding events related to SRIs have ranged from ecchymosis, hematoma, epistaxis, and petechiae to life-threatening hemorrhages. Additional epidemiological studies have confirmed the association between use of these agents and the occurrence of upper gastrointestinal bleeding, and concurrent use of NSAIDs or aspirin was found to potentiate the risk. Preliminary data also suggest that there may be a pharmacodynamic interaction between SSRIs and oral anticoagulants that can cause an increased bleeding diathesis. Concomitant administration of paroxetine and warfarin, specifically, has been associated with an increased frequency of bleeding without apparent changes in the disposition of either drug or changes in the prothrombin time. Bleeding has also been reported with fluoxetine and warfarin, while citalopram and sertraline have been reported to prolong the prothrombin time of patients taking warfarin by about 5% to 8%.
MANAGEMENT: Caution is advised if SRIs or clomipramine are used in combination with other drugs that affect hemostasis. Close clinical and laboratory observation for hematologic complications is recommended. Patients should be advised to promptly report any signs of bleeding to their physician, including pain, swelling, headache, dizziness, weakness, prolonged bleeding from cuts, increased menstrual flow, vaginal bleeding, nosebleeds, bleeding of gums from brushing, unusual bleeding or bruising, red or brown urine, or red or black stools.
morphine ↔ paroxetine
Applies to: Kadian (morphine), paroxetine
MONITOR: Central nervous system- and/or respiratory-depressant effects may be additively or synergistically increased in patients taking multiple drugs that cause these effects, especially in elderly or debilitated patients.
MANAGEMENT: During concomitant use of these drugs, patients should be monitored for potentially excessive or prolonged CNS and respiratory depression. Ambulatory patients should be counseled to avoid hazardous activities requiring mental alertness and motor coordination until they know how these agents affect them, and to notify their physician if they experience excessive or prolonged CNS effects that interfere with their normal activities.
oxycodone ↔ paroxetine
Applies to: acetaminophen/oxycodone, paroxetine
MONITOR: Coadministration of oxycodone with serotonin reuptake inhibitors has been associated with development of the serotonin syndrome. The mechanism of interaction is unknown. Unlike other analgesics such as phenylpiperidine opioids (e.g., meperidine) and tramadol, oxycodone is not known to possess serotonergic activity and has not previously been associated with the serotonin syndrome. The report describes a bone marrow transplant patient who developed severe tremors and visual hallucinations after he dramatically increased his dosage of oxycodone while on a stable dosage of sertraline and cyclosporine. Discontinuation of cyclosporine did not completely resolve his hallucinations and had no effect on the tremors after 72 hours, which led to consideration of a possible sertraline-oxycodone interaction. The patient's symptoms resolved after sertraline was withheld and cyproheptadine (a central serotonin antagonist) administered. Serotonin syndrome is a rare but serious and potentially fatal condition thought to result from hyperstimulation of brainstem 5-HT1A and 2A receptors. Symptoms of the serotonin syndrome may include mental status changes such as irritability, altered consciousness, confusion, hallucinations, and coma; autonomic dysfunction such as tachycardia, hyperthermia, diaphoresis, shivering, blood pressure lability, and mydriasis; neuromuscular abnormalities such as hyperreflexia, myoclonus, tremor, rigidity, and ataxia; and gastrointestinal symptoms such as abdominal cramping, nausea, vomiting, and diarrhea.
MANAGEMENT: Until more data are available, caution is advised if oxycodone is prescribed in combination with serotonin reuptake inhibitors, particularly in complicated patients such as transplant patients who are also receiving cyclosporine. Patients should be monitored for symptoms of the serotonin syndrome during treatment. Particular caution is advised when increasing the dosages of these agents. If serotonin syndrome develops or is suspected during the course of therapy, all serotonergic agents should be discontinued immediately and supportive care rendered as necessary. Moderately ill patients may also benefit from the administration of a serotonin antagonist (e.g., cyproheptadine, chlorpromazine). Severe cases should be managed under consultation with a toxicologist and may require sedation, neuromuscular paralysis, intubation, and mechanical ventilation in addition to the other measures. Patients should also be advised of potentially additive central nervous system effects from these agents and to avoid hazardous activities requiring complete mental alertness and motor coordination until they know how these agents affect them.
codeine ↔ losartan
Applies to: Ascomp with Codeine (aspirin/butalbital/caffeine/codeine), Cozaar (losartan)
MONITOR: Many psychotherapeutic and CNS-active agents (e.g., anxiolytics, sedatives, hypnotics, antidepressants, antipsychotics, opioids, alcohol, muscle relaxants) exhibit hypotensive effects, especially during initiation of therapy and dose escalation. Coadministration with antihypertensive agents, in particular vasodilators and alpha-blockers, may result in additive effects on blood pressure and orthostasis.
MANAGEMENT: Caution is advised during coadministration of these agents. Close monitoring for development of hypotension is recommended. Patients should be advised to avoid rising abruptly from a sitting or recumbent position and to notify their physician if they experience dizziness, lightheadedness, syncope, orthostasis, or tachycardia.
diazepam ↔ losartan
Applies to: diazepam, Cozaar (losartan)
MONITOR: Many psychotherapeutic and CNS-active agents (e.g., anxiolytics, sedatives, hypnotics, antidepressants, antipsychotics, opioids, alcohol, muscle relaxants) exhibit hypotensive effects, especially during initiation of therapy and dose escalation. Coadministration with antihypertensive agents, in particular vasodilators and alpha-blockers, may result in additive effects on blood pressure and orthostasis.
MANAGEMENT: Caution is advised during coadministration of these agents. Close monitoring for development of hypotension is recommended. Patients should be advised to avoid rising abruptly from a sitting or recumbent position and to notify their physician if they experience dizziness, lightheadedness, syncope, orthostasis, or tachycardia.
aspirin ↔ losartan
Applies to: Ascomp with Codeine (aspirin/butalbital/caffeine/codeine), Cozaar (losartan)
MONITOR: Nonsteroidal anti-inflammatory drugs (NSAIDs) may attenuate the antihypertensive effects of angiotensin II receptor antagonists. The proposed mechanism is NSAID-induced inhibition of renal prostaglandin synthesis, which results in unopposed pressor activity producing hypertension. In addition, NSAIDs can cause fluid retention, which also affects blood pressure. Clinical data are limited.
MONITOR: Concomitant use of NSAIDs and angiotensin II receptor antagonists may increase the risk of renal impairment, particularly in volume-depleted patients. Chronic use of NSAIDs alone may be associated with renal toxicities, including elevations in serum creatinine and BUN, tubular necrosis, glomerulitis, renal papillary necrosis, acute interstitial nephritis, nephrotic syndrome, and renal failure. Additionally, in patients with prerenal conditions whose renal perfusion may be dependent on the function of prostaglandins, NSAIDs may precipitate overt renal decompensation via a dose-related inhibition of prostaglandin synthesis. Angiotensin II receptor antagonists can further worsen renal function by blocking the effect of angiotensin II-mediated efferent arteriolar vasoconstriction, thereby decreasing glomerular filtration.
MANAGEMENT: Patients receiving angiotensin II receptor antagonists who require prolonged (greater than 1 week) concomitant therapy with an NSAID should have blood pressure monitored more closely following initiation, discontinuation, or change of dosage of the NSAID. Renal function should also be evaluated periodically during prolonged coadministration. The interaction is not expected to occur with low doses (e.g., low-dose aspirin) or intermittent short-term administration of NSAIDs.
morphine ↔ losartan
Applies to: Kadian (morphine), Cozaar (losartan)
MONITOR: Many psychotherapeutic and CNS-active agents (e.g., anxiolytics, sedatives, hypnotics, antidepressants, antipsychotics, opioids, alcohol, muscle relaxants) exhibit hypotensive effects, especially during initiation of therapy and dose escalation. Coadministration with antihypertensive agents, in particular vasodilators and alpha-blockers, may result in additive effects on blood pressure and orthostasis.
MANAGEMENT: Caution is advised during coadministration of these agents. Close monitoring for development of hypotension is recommended. Patients should be advised to avoid rising abruptly from a sitting or recumbent position and to notify their physician if they experience dizziness, lightheadedness, syncope, orthostasis, or tachycardia.
oxycodone ↔ losartan
Applies to: acetaminophen/oxycodone, Cozaar (losartan)
MONITOR: Many psychotherapeutic and CNS-active agents (e.g., anxiolytics, sedatives, hypnotics, antidepressants, antipsychotics, opioids, alcohol, muscle relaxants) exhibit hypotensive effects, especially during initiation of therapy and dose escalation. Coadministration with antihypertensive agents, in particular vasodilators and alpha-blockers, may result in additive effects on blood pressure and orthostasis.
MANAGEMENT: Caution is advised during coadministration of these agents. Close monitoring for development of hypotension is recommended. Patients should be advised to avoid rising abruptly from a sitting or recumbent position and to notify their physician if they experience dizziness, lightheadedness, syncope, orthostasis, or tachycardia.
morphine ↔ butalbital
Applies to: Kadian (morphine), Ascomp with Codeine (aspirin/butalbital/caffeine/codeine)
MONITOR: Central nervous system- and/or respiratory-depressant effects may be additively or synergistically increased in patients taking multiple drugs that cause these effects, especially in elderly or debilitated patients.
MANAGEMENT: During concomitant use of these drugs, patients should be monitored for potentially excessive or prolonged CNS and respiratory depression. Ambulatory patients should be counseled to avoid hazardous activities requiring mental alertness and motor coordination until they know how these agents affect them, and to notify their physician if they experience excessive or prolonged CNS effects that interfere with their normal activities.
levothyroxine ↔ butalbital
Applies to: levothyroxine, Ascomp with Codeine (aspirin/butalbital/caffeine/codeine)
MONITOR: Barbiturates may decrease exogenous thyroid plasma concentrations resulting in elevated thyroid stimulating hormone (TSH) and exacerbation of hypothyroidism. The mechanism may be induction of hepatic CYP450 enzymes responsible for thyroxine (T4) and triiodothyronine (T3) metabolism. Clinical data are limited.
MANAGEMENT: Clinical monitoring of patient response, including laboratory serum TSH concentrations, is recommended. Adjustment of thyroid replacement dosage may be indicated when initiating or discontinuing barbiturate therapy.
morphine ↔ oxycodone
Applies to: Kadian (morphine), acetaminophen/oxycodone
MONITOR: Central nervous system- and/or respiratory-depressant effects may be additively or synergistically increased in patients taking multiple drugs that cause these effects, especially in elderly or debilitated patients.
MANAGEMENT: During concomitant use of these drugs, patients should be monitored for potentially excessive or prolonged CNS and respiratory depression. Ambulatory patients should be counseled to avoid hazardous activities requiring mental alertness and motor coordination until they know how these agents affect them, and to notify their physician if they experience excessive or prolonged CNS effects that interfere with their normal activities.
codeine ↔ oxycodone
Applies to: Ascomp with Codeine (aspirin/butalbital/caffeine/codeine), acetaminophen/oxycodone
MONITOR: Central nervous system- and/or respiratory-depressant effects may be additively or synergistically increased in patients taking multiple drugs that cause these effects, especially in elderly or debilitated patients.
MANAGEMENT: During concomitant use of these drugs, patients should be monitored for potentially excessive or prolonged CNS and respiratory depression. Ambulatory patients should be counseled to avoid hazardous activities requiring mental alertness and motor coordination until they know how these agents affect them, and to notify their physician if they experience excessive or prolonged CNS effects that interfere with their normal activities.
diazepam ↔ morphine
Applies to: diazepam, Kadian (morphine)
MONITOR: Central nervous system- and/or respiratory-depressant effects may be additively or synergistically increased in patients taking multiple drugs that cause these effects, especially in elderly or debilitated patients.
MANAGEMENT: During concomitant use of these drugs, patients should be monitored for potentially excessive or prolonged CNS and respiratory depression. Ambulatory patients should be counseled to avoid hazardous activities requiring mental alertness and motor coordination until they know how these agents affect them, and to notify their physician if they experience excessive or prolonged CNS effects that interfere with their normal activities.
aspirin ↔ calcium carbonate
Applies to: Ascomp with Codeine (aspirin/butalbital/caffeine/codeine), Oysco 500 with D (calcium/vitamin d)
MONITOR: Chronic administration of antacids may reduce serum salicylate concentrations in patients receiving large doses of aspirin or other salicylates. The mechanism involves reduction in salicylate renal tubular reabsorption due to urinary alkalinization by antacids, resulting in increased renal salicylate clearance. In three children treated with large doses of aspirin for rheumatic fever, serum salicylate levels declined 30% to 70% during coadministration with a magnesium and aluminum hydroxide antacid. Other studies have found similar, albeit less dramatic results. Antacids reportedly have no effect on the oral bioavailability of aspirin in healthy adults. However, administration of antacids containing either aluminum and magnesium hydroxide or calcium carbonate two hours before aspirin dosing led to reduced absorption of aspirin in uremic patients.
MANAGEMENT: Patients treated chronically with antacids (or oral medications that contain antacids such as didanosine buffered tablets or pediatric oral solution) and large doses of salicylates (i.e. 3 g/day or more) should be monitored for potentially diminished or inadequate analgesic and anti-inflammatory effects, and the salicylate dosage adjusted if necessary.
levothyroxine ↔ calcium carbonate
Applies to: levothyroxine, Oysco 500 with D (calcium/vitamin d)
ADJUST DOSING INTERVAL: Concurrent administration of calcium-containing products may decrease the oral bioavailability of levothyroxine by one-third in some patients. Pharmacologic effects of levothyroxine may be reduced. The exact mechanism of interaction is unknown but may involve nonspecific adsorption of levothyroxine to calcium at acidic pH levels, resulting in an insoluble complex that is poorly absorbed from the gastrointestinal tract. In one study, 20 patients with hypothyroidism who were taking a stable long-term regimen of levothyroxine demonstrated modest but significant decreases in mean free and total thyroxine (T4) levels as well as a corresponding increase in mean thyrotropin (thyroid-stimulating hormone, or TSH) level following the addition of calcium carbonate (1200 mg/day of elemental calcium) for 3 months. Four patients had serum TSH levels that were higher than the normal range. Both T4 and TSH levels returned to near-baseline 2 months after discontinuation of calcium, which further supported the likelihood of an interaction. In addition, there have been case reports suggesting decreased efficacy of levothyroxine during calcium coadministration. It is not known whether this interaction occurs with other thyroid hormone preparations.
MANAGEMENT: Some experts recommend separating the times of administration of levothyroxine and calcium-containing preparations by at least 4 hours. Monitoring of serum TSH levels is recommended. Patients with gastrointestinal or malabsorption disorders may be at a greater risk of developing clinical or subclinical hypothyroidism due to this interaction.
methotrexate ↔ caffeine
Applies to: methotrexate, Cafergot (caffeine/ergotamine), Ascomp with Codeine (aspirin/butalbital/caffeine/codeine)
MONITOR: Limited data suggest that consumption of greater than 180 mg/day of caffeine may interfere with the efficacy of methotrexate (MTX) in patients with rheumatoid arthritis. The exact mechanism of interaction is unknown but may be related to the antagonistic effect of caffeine on adenosine receptors, as anti-inflammatory properties of MTX is thought to result from the accumulation of adenosine. In a study of 39 patients treated with MTX 7.5 mg/week (without folate supplementation) for 3 months, patients with high caffeine intake (more than 180 mg/day) experienced significantly less improvement in morning stiffness and joint pain from baseline than patients with low caffeine intake (more than 120 mg/day). There were no significant differences between the responses of patients with moderate caffeine intake (120 to 180 mg/day) and those of the other 2 groups. In an interview of 91 patients treated with MTX, 26% of patients who discontinued the drug were regular coffee drinkers compared to only 2% of those still receiving the drug. Because treatment failure was the reason for MTX discontinuation in 80% of patients who discontinued, the investigators suggested that caffeine may have interfered with MTX efficacy.
MANAGEMENT: Until further information is available, the potential for interaction should be considered in patients who consume substantial amounts of caffeine and caffeine-containing foods and are prescribed methotrexate for rheumatoid arthritis. It may be appropriate to limit caffeine intake if an interaction is suspected in cases of treatment failure.
codeine ↔ morphine
Applies to: Ascomp with Codeine (aspirin/butalbital/caffeine/codeine), Kadian (morphine)
MONITOR: Central nervous system- and/or respiratory-depressant effects may be additively or synergistically increased in patients taking multiple drugs that cause these effects, especially in elderly or debilitated patients.
MANAGEMENT: During concomitant use of these drugs, patients should be monitored for potentially excessive or prolonged CNS and respiratory depression. Ambulatory patients should be counseled to avoid hazardous activities requiring mental alertness and motor coordination until they know how these agents affect them, and to notify their physician if they experience excessive or prolonged CNS effects that interfere with their normal activities.
codeine ↔ diazepam
Applies to: Ascomp with Codeine (aspirin/butalbital/caffeine/codeine), diazepam
MONITOR: Central nervous system- and/or respiratory-depressant effects may be additively or synergistically increased in patients taking multiple drugs that cause these effects, especially in elderly or debilitated patients.
MANAGEMENT: During concomitant use of these drugs, patients should be monitored for potentially excessive or prolonged CNS and respiratory depression. Ambulatory patients should be counseled to avoid hazardous activities requiring mental alertness and motor coordination until they know how these agents affect them, and to notify their physician if they experience excessive or prolonged CNS effects that interfere with their normal activities.
acetaminophen ↔ butalbital
Applies to: acetaminophen/oxycodone, Ascomp with Codeine (aspirin/butalbital/caffeine/codeine)
MONITOR: Barbiturates may increase the hepatotoxic potential of acetaminophen and decrease its therapeutic effects. The mechanism may be related to accelerated CYP450 metabolism of acetaminophen with consequent increase in hepatotoxic metabolites. This interaction is of greatest concern in cases of acetaminophen overdose.
MANAGEMENT: Monitoring for altered efficacy and safety is recommended. Prolonged use or high doses of acetaminophen should be avoided by patients on barbiturate therapy.
diazepam ↔ oxycodone
Applies to: diazepam, acetaminophen/oxycodone
MONITOR: Central nervous system- and/or respiratory-depressant effects may be additively or synergistically increased in patients taking multiple drugs that cause these effects, especially in elderly or debilitated patients.
MANAGEMENT: During concomitant use of these drugs, patients should be monitored for potentially excessive or prolonged CNS and respiratory depression. Ambulatory patients should be counseled to avoid hazardous activities requiring mental alertness and motor coordination until they know how these agents affect them, and to notify their physician if they experience excessive or prolonged CNS effects that interfere with their normal activities.
butalbital ↔ paroxetine
Applies to:
Ascomp with Codeine (aspirin/butalbital/caffeine/codeine), paroxetine
Concomitant administration of paroxetine and barbiturates may decrease serum paroxetine levels and half-life. The proposed mechanism is induction of CYP450 hepatic metabolism by barbiturates. The clinical significance is unknown. Patients should be monitored for clinical response and the dose adjusted if an interaction is suspected.
diazepam ↔ calcium carbonate
Applies to: diazepam, Oysco 500 with D (calcium/vitamin d)
A number of studies have reported that antacids can delay the gastrointestinal absorption and reduce the peak plasma concentration (Cmax) of some benzodiazepines, including clorazepate, chlordiazepoxide and diazepam, although the overall extent of absorption is generally not affected. The exact mechanism of interaction is unknown but may involve delayed gastric emptying or cation binding of the benzodiazepine. As a result, benzodiazepine onset of action may be delayed and clinical effects diminished. However, one study reported a significant increase in diazepam absorption during coadministration with aluminum hydroxide, and there was a marginal increase in the onset of sedative effect. Aluminum hydroxide also increased the Cmax and systemic exposure (AUC) of triazolam in 11 dialysis patients such that their drug levels reached into the range observed for the matched controls. In contrast, another study by the same group of investigators found no significant effect of aluminum hydroxide on temazepam absorption or Cmax in 11 patients with end-stage renal disease. A multi-dose study also failed to find an effect of antacids on the steady-state levels of N-desmethyldiazepam, the active metabolite of clorazepate, although an acidic environment is thought to be necessary for the rapid conversion. Based on available data, the clinical significance of this interaction appears to be minor. As a precaution, patients may want to consider separating the administration times of benzodiazepines and antacids or oral medications that contain antacids (e.g., didanosine buffered tablets or pediatric oral solution) by 2 to 3 hours.
diazepam ↔ caffeine
Applies to: diazepam, Cafergot (caffeine/ergotamine), Ascomp with Codeine (aspirin/butalbital/caffeine/codeine)
One study has reported a 22% reduction in diazepam plasma levels when coadministered with caffeine. The exact mechanism of this interaction has not been specified. Physicians and patients should be aware that changes to caffeine consumption habits may impact the efficacy of diazepam therapy.
aspirin ↔ caffeine
Applies to:
Ascomp with Codeine (aspirin/butalbital/caffeine/codeine), Cafergot (caffeine/ergotamine)
One study has reported that coadministration of caffeine and aspirin lead to a 25% increase in the rate of appearance and 17% increase in maximum concentration of salicylate in the plasma. A significantly higher area under the plasma concentration time curve of salicylate was also reported when both drugs were administered together. The exact mechanism of this interaction has not been specified. Physicians and patients should be aware that coadministration of aspirin and caffeine may lead to higher salicylate levels faster.\
butalbital ↔ losartan
Applies to: Ascomp with Codeine (aspirin/butalbital/caffeine/codeine), Cozaar (losartan)
Concomitant administration of phenobarbital and losartan has been reported to decrease the area under the plasma concentration-time curve (AUC) of losartan and its active metabolite by as much as 20%. The mechanism of action is thought to be induction of losartan metabolism by phenobarbital. While data is available for phenobarbital only, theoretically this interaction may occur with other barbiturates. The reduction in the AUC was not considered significant, however, clinical monitoring for evidence of altered losartan effect is recommended if these drugs are used together.
No other interactions were found between your selected drugs.
Note: this does not necessarily mean no interactions exist. ALWAYS consult with your doctor or pharmacist.
Other drugs that your selected drugs interact with:
- acetaminophen/oxycodone interacts with more than 400 other drugs.
- diazepam interacts with more than 300 other drugs.
- levothyroxine interacts with more than 100 other drugs.
- loratadine interacts with more than 40 other drugs.
- methotrexate interacts with more than 300 other drugs.
- multivitamin interacts with 7 other drugs.
- paroxetine interacts with more than 400 other drugs.
- Ascomp with Codeine (aspirin/butalbital/caffeine/codeine) interacts with more than 1000 other drugs.
- Cafergot (caffeine/ergotamine) interacts with more than 200 other drugs.
- Cozaar (losartan) interacts with more than 200 other drugs.
- Kadian (morphine) interacts with more than 300 other drugs.
- Klor-Con (potassium chloride) interacts with more than 100 other drugs.
- Oysco 500 with D (calcium/vitamin d) interacts with more than 200 other drugs.
Interactions between your selected drugs and food
morphine ↔ food
Applies to: Kadian (morphine)
GENERALLY AVOID: The central nervous system-depressant effects of morphine and alcohol may be addictive. The combination may result in additive CNS-depression and impairment of judgment, thinking, and psychomotor skills. In more severe cases, respiratory depression, hypotension, profound sedation, and coma can occur.
GENERALLY AVOID: Consumption of alcohol while taking some sustained-release formulations of morphine may cause rapid release of the drug, resulting in high systemic levels of morphine that may be potentially lethal. Alcohol apparently can disrupt the release mechanism of some sustained-release formulations. The interaction was observed in in vitro studies using a 24-hour morphine formulation (Avinza 30 mg capsule, available in the U.S. from Ligand Pharmaceuticals). When the capsule was mixed with 900 mL of buffer solutions containing ethanol 20% and 40%, the dose of morphine that was released was alcohol concentration-dependent, leading to a more rapid release of morphine. Although the clinical relevance of this finding is unknown, 'dose-dumping' into the bloodstream is conceivable.
MANAGEMENT: Until more information is available, patients taking sustained-release formulations of morphine should not consume alcohol or use medications that contain alcohol. In general, potent narcotics such as morphine should not be combined with alcohol.
methotrexate ↔ food
Applies to: methotrexate
MONITOR: Limited data suggest that consumption of greater than 180 mg/day of caffeine may interfere with the efficacy of methotrexate (MTX) in patients with rheumatoid arthritis. The exact mechanism of interaction is unknown but may be related to the antagonistic effect of caffeine on adenosine receptors, as anti-inflammatory properties of MTX is thought to result from the accumulation of adenosine. In a study of 39 patients treated with MTX 7.5 mg/week (without folate supplementation) for 3 months, patients with high caffeine intake (more than 180 mg/day) experienced significantly less improvement in morning stiffness and joint pain from baseline than patients with low caffeine intake (more than 120 mg/day). There were no significant differences between the responses of patients with moderate caffeine intake (120 to 180 mg/day) and those of the other 2 groups. In an interview of 91 patients treated with MTX, 26% of patients who discontinued the drug were regular coffee drinkers compared to only 2% of those still receiving the drug. Because treatment failure was the reason for MTX discontinuation in 80% of patients who discontinued, the investigators suggested that caffeine may have interfered with MTX efficacy.
MANAGEMENT: Until further information is available, the potential for interaction should be considered in patients who consume substantial amounts of caffeine and caffeine-containing foods and are prescribed methotrexate for rheumatoid arthritis. It may be appropriate to limit caffeine intake if an interaction is suspected in cases of treatment failure.
diazepam ↔ food
Applies to: diazepam
MONITOR: Grapefruit juice may increase the plasma concentrations of some orally administered drugs that are primarily metabolized by the CYP450 3A4 isoenzyme. The proposed mechanism is inhibition of CYP450 3A4-mediated first-pass metabolism in the gut wall by certain compounds present in grapefruits. The extent and clinical significance of many of these interactions are unknown. Moreover, pharmacokinetic alterations associated with interactions involving grapefruit juice are often subject to a high degree of interpatient variability.
MANAGEMENT: Patients who regularly consume grapefruits and grapefruit juice should be monitored for adverse effects and altered plasma concentrations of drugs that are metabolized by CYP450 3A4. Grapefruits and grapefruit juice should be avoided if an interaction is suspected. Orange juice is not expected to interact with these drugs.
ergotamine ↔ food
Applies to: Cafergot (caffeine/ergotamine)
MONITOR: Grapefruit juice may increase the plasma concentrations of some orally administered drugs that are primarily metabolized by the CYP450 3A4 isoenzyme. The proposed mechanism is inhibition of CYP450 3A4-mediated first-pass metabolism in the gut wall by certain compounds present in grapefruits. The extent and clinical significance of many of these interactions are unknown. Moreover, pharmacokinetic alterations associated with interactions involving grapefruit juice are often subject to a high degree of interpatient variability.
MANAGEMENT: Patients who regularly consume grapefruits and grapefruit juice should be monitored for adverse effects and altered plasma concentrations of drugs that are metabolized by CYP450 3A4. Grapefruits and grapefruit juice should be avoided if an interaction is suspected. Orange juice is not expected to interact with these drugs.
levothyroxine ↔ food
Applies to: levothyroxine
ADJUST DOSING INTERVAL: Consumption of certain foods as well as the timing of meals relative to dosing may affect the absorption of T4 thyroid hormone (i.e., levothyroxine). T4 absorption is increased by fasting and decreased by foods such as soybean flour (e.g., infant formula), cotton seed meal, walnuts, dietary fiber, calcium, and calcium fortified juices.
MANAGEMENT: Preparations containing T4 thyroid hormone should be administered on a consistent schedule with regard to time of day and relation to meals so as to avoid large fluctuations in serum levels. Foods that may affect T4 absorption should be avoided within several hours of dosing if possible.
losartan ↔ food
Applies to: Cozaar (losartan)
GENERALLY AVOID: Moderate-to-high dietary intake of potassium, especially salt substitutes, may increase the risk of hyperkalemia in some patients who are using angiotensin II receptor blockers (ARBs). ARBs can promote hyperkalemia through inhibition of angiotensin II-induced aldosterone secretion. Patients with diabetes, heart failure, dehydration, or renal insufficiency have a greater risk of developing hyperkalemia.
MANAGEMENT: Patients should receive dietary counseling and be advised to not use potassium-containing salt substitutes or over-the-counter potassium supplements without consulting their physician. If salt substitutes are used concurrently, regular monitoring of serum potassium levels is recommended. Patients should also be advised to seek medical attention if they experience symptoms of hyperkalemia such as weakness, irregular heartbeat, confusion, tingling of the extremities, or feelings of heaviness in the legs.
MONITOR: Grapefruit juice may modestly decrease and delay the conversion of losartan to its active metabolite, E3174. The proposed mechanism is inhibition of CYP450 3A4-mediated first-pass metabolism in the gut wall by certain compounds present in grapefruits. The clinical significance is unknown. Moreover, pharmacokinetic alterations associated with interactions involving grapefruit juice are often subject to a high degree of interpatient variability.
MANAGEMENT: Patients who regularly consume grapefruits and grapefruit juice should be monitored for altered efficacy of losartan. Grapefruits and grapefruit juice should be avoided if an interaction is suspected. Orange juice is not expected to interact.
caffeine ↔ food
Applies to: Ascomp with Codeine (aspirin/butalbital/caffeine/codeine), Cafergot (caffeine/ergotamine)
The effect of grapefruit juice on the pharmacologic activity of caffeine is controversial. One report suggests that grapefruit juice increases the effect of caffeine. The proposed mechanism is inhibition of cytochrome P-450 metabolism of caffeine. However, a well-conducted pharmacokinetic/pharmacodynamic study did not demonstrate this effect. The clinical significance of this potential interaction is unknown.
loratadine ↔ food
Applies to: loratadine
Theoretically, grapefruit juice may increase the plasma concentrations of loratadine as it does other drugs that are substrates of the CYP450 3A4 enzymatic pathway. The proposed mechanism is inhibition of CYP450 3A4-mediated first-pass metabolism in the gut wall by certain compounds present in grapefruits. The clinical significance of this potential interaction is unknown. Reported interactions with potent CYP450 3A4 inhibitors like clarithromycin, erythromycin and ketoconazole have produced substantial increases in the area under the plasma concentration-time curve (AUC) of loratadine and its active metabolite, descarboethoxyloratadine, without associated changes in the overall safety profile of the drug.
_____________________________________________________
Drug Interactions Results
Drug interactions for the following 13 drug(s):
Unsaved Drug List |
|---|
| acetaminophen/oxycodone |
| diazepam |
| levothyroxine |
| loratadine |
| methotrexate |
| multivitamin |
| paroxetine |
| Ascomp with Codeine (aspirin/butalbital/caffeine/codeine) |
| Cafergot (caffeine/ergotamine) |
| Cozaar (losartan) |
| Kadian (morphine) |
| Klor-Con (potassium chloride) |
| Oysco 500 with D (calcium/vitamin d) |
Interactions between your selected drugs
potassium chloride ↔ losartan
Major Drug Interaction
Applies to: Klor-Con (potassium chloride), Cozaar (losartan)
MONITOR CLOSELY: Concomitant use of angiotensin II receptor blockers (ARBs) and potassium salts may increase the risk of hyperkalemia. Inhibition of angiotensin II results in decreased aldosterone secretion, which in turn causes potassium retention. Risk factors for developing severe or life-threatening hyperkalemia may include renal impairment, diabetes, old age, severe or worsening heart failure, dehydration, and concomitant use of other agents that block the renin-angiotensin-aldosterone system or otherwise increase serum potassium levels.
MANAGEMENT: Caution is advised if angiotensin II receptor blockers must be used concurrently with potassium salts, particularly in patients with renal impairment, diabetes, old age, severe or worsening heart failure, dehydration, or concomitant therapy with other agents that increase serum potassium such as nonsteroidal anti-inflammatory drugs, beta-blockers, cyclosporine, heparin, tacrolimus, trimethoprim, and licorice. The combination should generally be avoided in these patients unless absolutely necessary and the benefits outweigh the potential risks. Serum potassium and renal function should be checked prior to initiating therapy and regularly thereafter. Patients should be given counseling on the appropriate levels of potassium and fluid intake, and advised to seek medical attention if they experience signs and symptoms of hyperkalemia such as nausea, vomiting, weakness, listlessness, tingling of the extremities, paralysis, confusion, weak pulse, and a slow or irregular heartbeat.
methotrexate ↔ aspirin
Major Drug Interaction
Applies to: methotrexate, Ascomp with Codeine (aspirin/butalbital/caffeine/codeine)
GENERALLY AVOID: Salicylates may interfere with the renal elimination of methotrexate and may displace it from binding sites. The pharmacologic effect and toxicity of methotrexate may be increased. Patients receiving high-dose methotrexate are at a greater risk of developing toxicity.
MANAGEMENT: If these agents must be used concomitantly, caution should be exercised and the patient should be monitored closely for signs and symptoms of bone marrow suppression and nephrotoxicity. Patients should be advised to report possible symptoms of toxicity including nausea, vomiting, diarrhea, stomatitis, sore throat, chills, fever, rash, unusual bruising or bleeding, jaundice, dark urine, swelling of the extremities, or shortness of breath to their physician. Patients should also be counseled to avoid any other over-the-counter salicylate products.
ergotamine ↔ paroxetine
Major Drug Interaction
Applies to: Cafergot (caffeine/ergotamine), paroxetine
MONITOR CLOSELY: Concomitant use of agents with serotonergic activity such as serotonin reuptake inhibitors, monoamine oxidase inhibitors, tricyclic antidepressants, 5-HT1 receptor agonists, ergot alkaloids, lithium, St. John's wort, phenylpiperidine opioids, dextromethorphan, and tryptophan may potentiate the risk of serotonin syndrome, which is a rare but serious and potentially fatal condition thought to result from hyperstimulation of brainstem 5-HT1A and 2A receptors. Symptoms of the serotonin syndrome may include mental status changes such as irritability, altered consciousness, confusion, hallucinations, and coma; autonomic dysfunction such as tachycardia, hyperthermia, diaphoresis, shivering, blood pressure lability, and mydriasis; neuromuscular abnormalities such as hyperreflexia, myoclonus, tremor, rigidity, and ataxia; and gastrointestinal symptoms such as abdominal cramping, nausea, vomiting, and diarrhea.
MANAGEMENT: In general, the concomitant use of multiple serotonergic agents should be avoided if possible, or otherwise approached with caution if potential benefit is deemed to outweigh the risk. Patients should be closely monitored for symptoms of the serotonin syndrome during treatment. Particular caution is advised when increasing the dosages of these agents. The potential risk for serotonin syndrome should be considered even when administering serotonergic agents sequentially, as some agents may demonstrate a prolonged elimination half-life. For example, a 5-week washout period is recommended following use of fluoxetine before administering another serotonergic agent. If serotonin syndrome develops or is suspected during the course of therapy, all serotonergic agents should be discontinued immediately and supportive care rendered as necessary. Moderately ill patients may also benefit from the administration of a serotonin antagonist (e.g., cyproheptadine, chlorpromazine). Severe cases should be managed under consultation with a toxicologist and may require sedation, neuromuscular paralysis, intubation, and mechanical ventilation in addition to the other measures.
acetaminophen ↔ methotrexate
Moderate Drug Interaction
Applies to: acetaminophen/oxycodone, methotrexate
GENERALLY AVOID: Coadministration of methotrexate with other agents known to induce hepatotoxicity may potentiate the risk of liver injury. Methotrexate, especially at higher doses or with prolonged treatment, has been associated with hepatotoxicity including acute hepatitis, chronic fibrosis, necrosis, cirrhosis, and liver enzyme elevations.
MANAGEMENT: The use of methotrexate in combination with other potentially hepatotoxic agents (e.g., acetaminophen; alcohol; androgens and anabolic steroids; antituberculous agents; azole antifungal agents; ACE inhibitors; endothelin receptor antagonists; interferons; nucleoside reverse transcriptase inhibitors; retinoids; thiazolidinediones; anticonvulsants such as carbamazepine, hydantoins, felbamate, and valproic acid; lipid-lowering medications such as fenofibrate, HMG-CoA reductase inhibitors, and niacin; herbals and nutritional supplements such as black cohosh, chaparral, comfrey, DHEA, kava, pennyroyal oil, and red yeast rice) is generally not recommended unless the potential benefit outweighs the risk. Patients treated with methotrexate should be advised to seek medical attention if they experience potential signs and symptoms of hepatotoxicity such as fever, rash, itching, anorexia, nausea, vomiting, fatigue, right upper quadrant pain, dark urine, light-colored stools, and jaundice. Baseline and regular monitoring of hepatic function is recommended.
oxycodone ↔ butalbital
Moderate Drug Interaction
Applies to: acetaminophen/oxycodone, Ascomp with Codeine (aspirin/butalbital/caffeine/codeine)
MONITOR: Central nervous system- and/or respiratory-depressant effects may be additively or synergistically increased in patients taking multiple drugs that cause these effects, especially in elderly or debilitated patients.
MANAGEMENT: During concomitant use of these drugs, patients should be monitored for potentially excessive or prolonged CNS and respiratory depression. Ambulatory patients should be counseled to avoid hazardous activities requiring mental alertness and motor coordination until they know how these agents affect them, and to notify their physician if they experience excessive or prolonged CNS effects that interfere with their normal activities.
butalbital ↔ ergocalciferol
Moderate Drug Interaction
Applies to: Ascomp with Codeine (aspirin/butalbital/caffeine/codeine), Oysco 500 with D (calcium/vitamin d)
MONITOR: Coadministration with CYP450 inducers such as rifampin, isoniazid, barbiturates, and certain anticonvulsants may decrease the pharmacologic effects of vitamin D analogs. These agents are thought to induce the hepatic conversion of vitamin D to inactive metabolites and have been shown to reduce circulating levels of active vitamin D, sometimes accompanied by reduced serum calcium and increased parathyroid hormone levels. Patients on long-term anticonvulsant therapy have occasionally developed osteomalacia, presumably due to interference with vitamin D and calcium metabolism. There have also been isolated reports of patients who responded poorly to vitamin D supplements during treatment with phenytoin and/or primidone.
MANAGEMENT: Patients receiving vitamin D analogs with CYP450 inducers should be monitored for potentially reduced vitamin D effects. Dosage adjustments may be necessary.
codeine ↔ paroxetine
Moderate Drug Interaction
Applies to: Ascomp with Codeine (aspirin/butalbital/caffeine/codeine), paroxetine
MONITOR: Drugs that are inhibitors of CYP450 2D6 may interfere with the analgesic effect of codeine. The mechanism is decreased in vivo conversion of codeine to morphine, a metabolic reaction mediated by CYP450 2D6.
MANAGEMENT: The possibility of reduced or inadequate pain relief should be considered in patients receiving codeine with drugs that inhibit CYP450 2D6. An increase in the codeine dosage or a different analgesic agent may be necessary in patients requiring therapy with CYP450 2D6 inhibitors.
diazepam ↔ paroxetine
Moderate Drug Interaction
Applies to: diazepam, paroxetine
MONITOR: Central nervous system- and/or respiratory-depressant effects may be additively or synergistically increased in patients taking multiple drugs that cause these effects, especially in elderly or debilitated patients.
MANAGEMENT: During concomitant use of these drugs, patients should be monitored for potentially excessive or prolonged CNS and respiratory depression. Ambulatory patients should be counseled to avoid hazardous activities requiring mental alertness and motor coordination until they know how these agents affect them, and to notify their physician if they experience excessive or prolonged CNS effects that interfere with their normal activities.
aspirin ↔ paroxetine
Moderate Drug Interaction
Applies to: Ascomp with Codeine (aspirin/butalbital/caffeine/codeine), paroxetine
MONITOR: Serotonin reuptake inhibitors (SRIs) may potentiate the risk of bleeding in patients treated with ulcerogenic agents and agents that affect hemostasis such as anticoagulants, platelet inhibitors, thrombin inhibitors, thrombolytic agents, or agents that commonly cause thrombocytopenia. The tricyclic antidepressant, clomipramine, is also a strong SRI and may interact similarly. Serotonin release by platelets plays an important role in hemostasis, thus SRIs may alter platelet function and induce bleeding. Published case reports have documented the occurrence of bleeding episodes in patients treated with psychotropic agents that interfere with serotonin reuptake. Bleeding events related to SRIs have ranged from ecchymosis, hematoma, epistaxis, and petechiae to life-threatening hemorrhages. Additional epidemiological studies have confirmed the association between use of these agents and the occurrence of upper gastrointestinal bleeding, and concurrent use of NSAIDs or aspirin was found to potentiate the risk. Preliminary data also suggest that there may be a pharmacodynamic interaction between SSRIs and oral anticoagulants that can cause an increased bleeding diathesis. Concomitant administration of paroxetine and warfarin, specifically, has been associated with an increased frequency of bleeding without apparent changes in the disposition of either drug or changes in the prothrombin time. Bleeding has also been reported with fluoxetine and warfarin, while citalopram and sertraline have been reported to prolong the prothrombin time of patients taking warfarin by about 5% to 8%.
MANAGEMENT: Caution is advised if SRIs or clomipramine are used in combination with other drugs that affect hemostasis. Close clinical and laboratory observation for hematologic complications is recommended. Patients should be advised to promptly report any signs of bleeding to their physician, including pain, swelling, headache, dizziness, weakness, prolonged bleeding from cuts, increased menstrual flow, vaginal bleeding, nosebleeds, bleeding of gums from brushing, unusual bleeding or bruising, red or brown urine, or red or black stools.
morphine ↔ paroxetine
Moderate Drug Interaction
Applies to: Kadian (morphine), paroxetine
MONITOR: Central nervous system- and/or respiratory-depressant effects may be additively or synergistically increased in patients taking multiple drugs that cause these effects, especially in elderly or debilitated patients.
MANAGEMENT: During concomitant use of these drugs, patients should be monitored for potentially excessive or prolonged CNS and respiratory depression. Ambulatory patients should be counseled to avoid hazardous activities requiring mental alertness and motor coordination until they know how these agents affect them, and to notify their physician if they experience excessive or prolonged CNS effects that interfere with their normal activities.
oxycodone ↔ paroxetine
Moderate Drug Interaction
Applies to: acetaminophen/oxycodone, paroxetine
MONITOR: Coadministration of oxycodone with serotonin reuptake inhibitors has been associated with development of the serotonin syndrome. The mechanism of interaction is unknown. Unlike other analgesics such as phenylpiperidine opioids (e.g., meperidine) and tramadol, oxycodone is not known to possess serotonergic activity and has not previously been associated with the serotonin syndrome. The report describes a bone marrow transplant patient who developed severe tremors and visual hallucinations after he dramatically increased his dosage of oxycodone while on a stable dosage of sertraline and cyclosporine. Discontinuation of cyclosporine did not completely resolve his hallucinations and had no effect on the tremors after 72 hours, which led to consideration of a possible sertraline-oxycodone interaction. The patient's symptoms resolved after sertraline was withheld and cyproheptadine (a central serotonin antagonist) administered. Serotonin syndrome is a rare but serious and potentially fatal condition thought to result from hyperstimulation of brainstem 5-HT1A and 2A receptors. Symptoms of the serotonin syndrome may include mental status changes such as irritability, altered consciousness, confusion, hallucinations, and coma; autonomic dysfunction such as tachycardia, hyperthermia, diaphoresis, shivering, blood pressure lability, and mydriasis; neuromuscular abnormalities such as hyperreflexia, myoclonus, tremor, rigidity, and ataxia; and gastrointestinal symptoms such as abdominal cramping, nausea, vomiting, and diarrhea.
MANAGEMENT: Until more data are available, caution is advised if oxycodone is prescribed in combination with serotonin reuptake inhibitors, particularly in complicated patients such as transplant patients who are also receiving cyclosporine. Patients should be monitored for symptoms of the serotonin syndrome during treatment. Particular caution is advised when increasing the dosages of these agents. If serotonin syndrome develops or is suspected during the course of therapy, all serotonergic agents should be discontinued immediately and supportive care rendered as necessary. Moderately ill patients may also benefit from the administration of a serotonin antagonist (e.g., cyproheptadine, chlorpromazine). Severe cases should be managed under consultation with a toxicologist and may require sedation, neuromuscular paralysis, intubation, and mechanical ventilation in addition to the other measures. Patients should also be advised of potentially additive central nervous system effects from these agents and to avoid hazardous activities requiring complete mental alertness and motor coordination until they know how these agents affect them.
codeine ↔ losartan
Moderate Drug Interaction
Applies to: Ascomp with Codeine (aspirin/butalbital/caffeine/codeine), Cozaar (losartan)
MONITOR: Many psychotherapeutic and CNS-active agents (e.g., anxiolytics, sedatives, hypnotics, antidepressants, antipsychotics, opioids, alcohol, muscle relaxants) exhibit hypotensive effects, especially during initiation of therapy and dose escalation. Coadministration with antihypertensive agents, in particular vasodilators and alpha-blockers, may result in additive effects on blood pressure and orthostasis.
MANAGEMENT: Caution is advised during coadministration of these agents. Close monitoring for development of hypotension is recommended. Patients should be advised to avoid rising abruptly from a sitting or recumbent position and to notify their physician if they experience dizziness, lightheadedness, syncope, orthostasis, or tachycardia.
diazepam ↔ losartan
Moderate Drug Interaction
Applies to: diazepam, Cozaar (losartan)
MONITOR: Many psychotherapeutic and CNS-active agents (e.g., anxiolytics, sedatives, hypnotics, antidepressants, antipsychotics, opioids, alcohol, muscle relaxants) exhibit hypotensive effects, especially during initiation of therapy and dose escalation. Coadministration with antihypertensive agents, in particular vasodilators and alpha-blockers, may result in additive effects on blood pressure and orthostasis.
MANAGEMENT: Caution is advised during coadministration of these agents. Close monitoring for development of hypotension is recommended. Patients should be advised to avoid rising abruptly from a sitting or recumbent position and to notify their physician if they experience dizziness, lightheadedness, syncope, orthostasis, or tachycardia.
aspirin ↔ losartan
Moderate Drug Interaction
Applies to: Ascomp with Codeine (aspirin/butalbital/caffeine/codeine), Cozaar (losartan)
MONITOR: Nonsteroidal anti-inflammatory drugs (NSAIDs) may attenuate the antihypertensive effects of angiotensin II receptor antagonists. The proposed mechanism is NSAID-induced inhibition of renal prostaglandin synthesis, which results in unopposed pressor activity producing hypertension. In addition, NSAIDs can cause fluid retention, which also affects blood pressure. Clinical data are limited.
MONITOR: Concomitant use of NSAIDs and angiotensin II receptor antagonists may increase the risk of renal impairment, particularly in volume-depleted patients. Chronic use of NSAIDs alone may be associated with renal toxicities, including elevations in serum creatinine and BUN, tubular necrosis, glomerulitis, renal papillary necrosis, acute interstitial nephritis, nephrotic syndrome, and renal failure. Additionally, in patients with prerenal conditions whose renal perfusion may be dependent on the function of prostaglandins, NSAIDs may precipitate overt renal decompensation via a dose-related inhibition of prostaglandin synthesis. Angiotensin II receptor antagonists can further worsen renal function by blocking the effect of angiotensin II-mediated efferent arteriolar vasoconstriction, thereby decreasing glomerular filtration.
MANAGEMENT: Patients receiving angiotensin II receptor antagonists who require prolonged (greater than 1 week) concomitant therapy with an NSAID should have blood pressure monitored more closely following initiation, discontinuation, or change of dosage of the NSAID. Renal function should also be evaluated periodically during prolonged coadministration. The interaction is not expected to occur with low doses (e.g., low-dose aspirin) or intermittent short-term administration of NSAIDs.
morphine ↔ losartan
Moderate Drug Interaction
Applies to: Kadian (morphine), Cozaar (losartan)
MONITOR: Many psychotherapeutic and CNS-active agents (e.g., anxiolytics, sedatives, hypnotics, antidepressants, antipsychotics, opioids, alcohol, muscle relaxants) exhibit hypotensive effects, especially during initiation of therapy and dose escalation. Coadministration with antihypertensive agents, in particular vasodilators and alpha-blockers, may result in additive effects on blood pressure and orthostasis.
MANAGEMENT: Caution is advised during coadministration of these agents. Close monitoring for development of hypotension is recommended. Patients should be advised to avoid rising abruptly from a sitting or recumbent position and to notify their physician if they experience dizziness, lightheadedness, syncope, orthostasis, or tachycardia.
oxycodone ↔ losartan
Moderate Drug Interaction
Applies to: acetaminophen/oxycodone, Cozaar (losartan)
MONITOR: Many psychotherapeutic and CNS-active agents (e.g., anxiolytics, sedatives, hypnotics, antidepressants, antipsychotics, opioids, alcohol, muscle relaxants) exhibit hypotensive effects, especially during initiation of therapy and dose escalation. Coadministration with antihypertensive agents, in particular vasodilators and alpha-blockers, may result in additive effects on blood pressure and orthostasis.
MANAGEMENT: Caution is advised during coadministration of these agents. Close monitoring for development of hypotension is recommended. Patients should be advised to avoid rising abruptly from a sitting or recumbent position and to notify their physician if they experience dizziness, lightheadedness, syncope, orthostasis, or tachycardia.
morphine ↔ butalbital
Moderate Drug Interaction
Applies to: Kadian (morphine), Ascomp with Codeine (aspirin/butalbital/caffeine/codeine)
MONITOR: Central nervous system- and/or respiratory-depressant effects may be additively or synergistically increased in patients taking multiple drugs that cause these effects, especially in elderly or debilitated patients.
MANAGEMENT: During concomitant use of these drugs, patients should be monitored for potentially excessive or prolonged CNS and respiratory depression. Ambulatory patients should be counseled to avoid hazardous activities requiring mental alertness and motor coordination until they know how these agents affect them, and to notify their physician if they experience excessive or prolonged CNS effects that interfere with their normal activities.
levothyroxine ↔ butalbital
Moderate Drug Interaction
Applies to: levothyroxine, Ascomp with Codeine (aspirin/butalbital/caffeine/codeine)
MONITOR: Barbiturates may decrease exogenous thyroid plasma concentrations resulting in elevated thyroid stimulating hormone (TSH) and exacerbation of hypothyroidism. The mechanism may be induction of hepatic CYP450 enzymes responsible for thyroxine (T4) and triiodothyronine (T3) metabolism. Clinical data are limited.
MANAGEMENT: Clinical monitoring of patient response, including laboratory serum TSH concentrations, is recommended. Adjustment of thyroid replacement dosage may be indicated when initiating or discontinuing barbiturate therapy.
morphine ↔ oxycodone
Moderate Drug Interaction
Applies to: Kadian (morphine), acetaminophen/oxycodone
MONITOR: Central nervous system- and/or respiratory-depressant effects may be additively or synergistically increased in patients taking multiple drugs that cause these effects, especially in elderly or debilitated patients.
MANAGEMENT: During concomitant use of these drugs, patients should be monitored for potentially excessive or prolonged CNS and respiratory depression. Ambulatory patients should be counseled to avoid hazardous activities requiring mental alertness and motor coordination until they know how these agents affect them, and to notify their physician if they experience excessive or prolonged CNS effects that interfere with their normal activities.
codeine ↔ oxycodone
Moderate Drug Interaction
Applies to: Ascomp with Codeine (aspirin/butalbital/caffeine/codeine), acetaminophen/oxycodone
MONITOR: Central nervous system- and/or respiratory-depressant effects may be additively or synergistically increased in patients taking multiple drugs that cause these effects, especially in elderly or debilitated patients.
MANAGEMENT: During concomitant use of these drugs, patients should be monitored for potentially excessive or prolonged CNS and respiratory depression. Ambulatory patients should be counseled to avoid hazardous activities requiring mental alertness and motor coordination until they know how these agents affect them, and to notify their physician if they experience excessive or prolonged CNS effects that interfere with their normal activities.
diazepam ↔ morphine
Moderate Drug Interaction
Applies to: diazepam, Kadian (morphine)
MONITOR: Central nervous system- and/or respiratory-depressant effects may be additively or synergistically increased in patients taking multiple drugs that cause these effects, especially in elderly or debilitated patients.
MANAGEMENT: During concomitant use of these drugs, patients should be monitored for potentially excessive or prolonged CNS and respiratory depression. Ambulatory patients should be counseled to avoid hazardous activities requiring mental alertness and motor coordination until they know how these agents affect them, and to notify their physician if they experience excessive or prolonged CNS effects that interfere with their normal activities.
aspirin ↔ calcium carbonate
Moderate Drug Interaction
Applies to: Ascomp with Codeine (aspirin/butalbital/caffeine/codeine), Oysco 500 with D (calcium/vitamin d)
MONITOR: Chronic administration of antacids may reduce serum salicylate concentrations in patients receiving large doses of aspirin or other salicylates. The mechanism involves reduction in salicylate renal tubular reabsorption due to urinary alkalinization by antacids, resulting in increased renal salicylate clearance. In three children treated with large doses of aspirin for rheumatic fever, serum salicylate levels declined 30% to 70% during coadministration with a magnesium and aluminum hydroxide antacid. Other studies have found similar, albeit less dramatic results. Antacids reportedly have no effect on the oral bioavailability of aspirin in healthy adults. However, administration of antacids containing either aluminum and magnesium hydroxide or calcium carbonate two hours before aspirin dosing led to reduced absorption of aspirin in uremic patients.
MANAGEMENT: Patients treated chronically with antacids (or oral medications that contain antacids such as didanosine buffered tablets or pediatric oral solution) and large doses of salicylates (i.e. 3 g/day or more) should be monitored for potentially diminished or inadequate analgesic and anti-inflammatory effects, and the salicylate dosage adjusted if necessary.
levothyroxine ↔ calcium carbonate
Moderate Drug Interaction
Applies to: levothyroxine, Oysco 500 with D (calcium/vitamin d)
ADJUST DOSING INTERVAL: Concurrent administration of calcium-containing products may decrease the oral bioavailability of levothyroxine by one-third in some patients. Pharmacologic effects of levothyroxine may be reduced. The exact mechanism of interaction is unknown but may involve nonspecific adsorption of levothyroxine to calcium at acidic pH levels, resulting in an insoluble complex that is poorly absorbed from the gastrointestinal tract. In one study, 20 patients with hypothyroidism who were taking a stable long-term regimen of levothyroxine demonstrated modest but significant decreases in mean free and total thyroxine (T4) levels as well as a corresponding increase in mean thyrotropin (thyroid-stimulating hormone, or TSH) level following the addition of calcium carbonate (1200 mg/day of elemental calcium) for 3 months. Four patients had serum TSH levels that were higher than the normal range. Both T4 and TSH levels returned to near-baseline 2 months after discontinuation of calcium, which further supported the likelihood of an interaction. In addition, there have been case reports suggesting decreased efficacy of levothyroxine during calcium coadministration. It is not known whether this interaction occurs with other thyroid hormone preparations.
MANAGEMENT: Some experts recommend separating the times of administration of levothyroxine and calcium-containing preparations by at least 4 hours. Monitoring of serum TSH levels is recommended. Patients with gastrointestinal or malabsorption disorders may be at a greater risk of developing clinical or subclinical hypothyroidism due to this interaction.
methotrexate ↔ caffeine
Moderate Drug Interaction
Applies to: methotrexate, Cafergot (caffeine/ergotamine), Ascomp with Codeine (aspirin/butalbital/caffeine/codeine)
MONITOR: Limited data suggest that consumption of greater than 180 mg/day of caffeine may interfere with the efficacy of methotrexate (MTX) in patients with rheumatoid arthritis. The exact mechanism of interaction is unknown but may be related to the antagonistic effect of caffeine on adenosine receptors, as anti-inflammatory properties of MTX is thought to result from the accumulation of adenosine. In a study of 39 patients treated with MTX 7.5 mg/week (without folate supplementation) for 3 months, patients with high caffeine intake (more than 180 mg/day) experienced significantly less improvement in morning stiffness and joint pain from baseline than patients with low caffeine intake (more than 120 mg/day). There were no significant differences between the responses of patients with moderate caffeine intake (120 to 180 mg/day) and those of the other 2 groups. In an interview of 91 patients treated with MTX, 26% of patients who discontinued the drug were regular coffee drinkers compared to only 2% of those still receiving the drug. Because treatment failure was the reason for MTX discontinuation in 80% of patients who discontinued, the investigators suggested that caffeine may have interfered with MTX efficacy.
MANAGEMENT: Until further information is available, the potential for interaction should be considered in patients who consume substantial amounts of caffeine and caffeine-containing foods and are prescribed methotrexate for rheumatoid arthritis. It may be appropriate to limit caffeine intake if an interaction is suspected in cases of treatment failure.
codeine ↔ morphine
Moderate Drug Interaction
Applies to: Ascomp with Codeine (aspirin/butalbital/caffeine/codeine), Kadian (morphine)
MONITOR: Central nervous system- and/or respiratory-depressant effects may be additively or synergistically increased in patients taking multiple drugs that cause these effects, especially in elderly or debilitated patients.
MANAGEMENT: During concomitant use of these drugs, patients should be monitored for potentially excessive or prolonged CNS and respiratory depression. Ambulatory patients should be counseled to avoid hazardous activities requiring mental alertness and motor coordination until they know how these agents affect them, and to notify their physician if they experience excessive or prolonged CNS effects that interfere with their normal activities.
codeine ↔ diazepam
Moderate Drug Interaction
Applies to: Ascomp with Codeine (aspirin/butalbital/caffeine/codeine), diazepam
MONITOR: Central nervous system- and/or respiratory-depressant effects may be additively or synergistically increased in patients taking multiple drugs that cause these effects, especially in elderly or debilitated patients.
MANAGEMENT: During concomitant use of these drugs, patients should be monitored for potentially excessive or prolonged CNS and respiratory depression. Ambulatory patients should be counseled to avoid hazardous activities requiring mental alertness and motor coordination until they know how these agents affect them, and to notify their physician if they experience excessive or prolonged CNS effects that interfere with their normal activities.
acetaminophen ↔ butalbital
Moderate Drug Interaction
Applies to: acetaminophen/oxycodone, Ascomp with Codeine (aspirin/butalbital/caffeine/codeine)
MONITOR: Barbiturates may increase the hepatotoxic potential of acetaminophen and decrease its therapeutic effects. The mechanism may be related to accelerated CYP450 metabolism of acetaminophen with consequent increase in hepatotoxic metabolites. This interaction is of greatest concern in cases of acetaminophen overdose.
MANAGEMENT: Monitoring for altered efficacy and safety is recommended. Prolonged use or high doses of acetaminophen should be avoided by patients on barbiturate therapy.
diazepam ↔ oxycodone
Moderate Drug Interaction
Applies to: diazepam, acetaminophen/oxycodone
MONITOR: Central nervous system- and/or respiratory-depressant effects may be additively or synergistically increased in patients taking multiple drugs that cause these effects, especially in elderly or debilitated patients.
MANAGEMENT: During concomitant use of these drugs, patients should be monitored for potentially excessive or prolonged CNS and respiratory depression. Ambulatory patients should be counseled to avoid hazardous activities requiring mental alertness and motor coordination until they know how these agents affect them, and to notify their physician if they experience excessive or prolonged CNS effects that interfere with their normal activities.
butalbital ↔ paroxetine
Minor Drug Interaction
Applies to: Ascomp with Codeine (aspirin/butalbital/caffeine/codeine), paroxetine
Concomitant administration of paroxetine and barbiturates may decrease serum paroxetine levels and half-life. The proposed mechanism is induction of CYP450 hepatic metabolism by barbiturates. The clinical significance is unknown. Patients should be monitored for clinical response and the dose adjusted if an interaction is suspected.
diazepam ↔ calcium carbonate
Minor Drug Interaction
Applies to: diazepam, Oysco 500 with D (calcium/vitamin d)
A number of studies have reported that antacids can delay the gastrointestinal absorption and reduce the peak plasma concentration (Cmax) of some benzodiazepines, including clorazepate, chlordiazepoxide and diazepam, although the overall extent of absorption is generally not affected. The exact mechanism of interaction is unknown but may involve delayed gastric emptying or cation binding of the benzodiazepine. As a result, benzodiazepine onset of action may be delayed and clinical effects diminished. However, one study reported a significant increase in diazepam absorption during coadministration with aluminum hydroxide, and there was a marginal increase in the onset of sedative effect. Aluminum hydroxide also increased the Cmax and systemic exposure (AUC) of triazolam in 11 dialysis patients such that their drug levels reached into the range observed for the matched controls. In contrast, another study by the same group of investigators found no significant effect of aluminum hydroxide on temazepam absorption or Cmax in 11 patients with end-stage renal disease. A multi-dose study also failed to find an effect of antacids on the steady-state levels of N-desmethyldiazepam, the active metabolite of clorazepate, although an acidic environment is thought to be necessary for the rapid conversion. Based on available data, the clinical significance of this interaction appears to be minor. As a precaution, patients may want to consider separating the administration times of benzodiazepines and antacids or oral medications that contain antacids (e.g., didanosine buffered tablets or pediatric oral solution) by 2 to 3 hours.
diazepam ↔ caffeine
Minor Drug Interaction
Applies to: diazepam, Cafergot (caffeine/ergotamine), Ascomp with Codeine (aspirin/butalbital/caffeine/codeine)
One study has reported a 22% reduction in diazepam plasma levels when coadministered with caffeine. The exact mechanism of this interaction has not been specified. Physicians and patients should be aware that changes to caffeine consumption habits may impact the efficacy of diazepam therapy.
aspirin ↔ caffeine
Minor Drug Interaction
Applies to: Ascomp with Codeine (aspirin/butalbital/caffeine/codeine), Cafergot (caffeine/ergotamine)
One study has reported that coadministration of caffeine and aspirin lead to a 25% increase in the rate of appearance and 17% increase in maximum concentration of salicylate in the plasma. A significantly higher area under the plasma concentration time curve of salicylate was also reported when both drugs were administered together. The exact mechanism of this interaction has not been specified. Physicians and patients should be aware that coadministration of aspirin and caffeine may lead to higher salicylate levels faster.
butalbital ↔ losartan
Minor Drug Interaction
Applies to: Ascomp with Codeine (aspirin/butalbital/caffeine/codeine), Cozaar (losartan)
Concomitant administration of phenobarbital and losartan has been reported to decrease the area under the plasma concentration-time curve (AUC) of losartan and its active metabolite by as much as 20%. The mechanism of action is thought to be induction of losartan metabolism by phenobarbital. While data is available for phenobarbital only, theoretically this interaction may occur with other barbiturates. The reduction in the AUC was not considered significant, however, clinical monitoring for evidence of altered losartan effect is recommended if these drugs are used together.
No other interactions were found between your selected drugs.
Note: this does not necessarily mean no interactions exist.
ALWAYS consult with your doctor or pharmacist.
Other drugs that your selected drugs interact with
- acetaminophen/oxycodone interacts with more than 400 other drugs.
- diazepam interacts with more than 300 other drugs.
- levothyroxine interacts with more than 100 other drugs.
- loratadine interacts with more than 40 other drugs.
- methotrexate interacts with more than 300 other drugs.
- multivitamin interacts with 7 other drugs.
- paroxetine interacts with more than 400 other drugs.
- Ascomp with Codeine (aspirin/butalbital/caffeine/codeine) interacts with more than 1000 other drugs.
- Cafergot (caffeine/ergotamine) interacts with more than 200 other drugs.
- Cozaar (losartan) interacts with more than 200 other drugs.
- Kadian (morphine) interacts with more than 300 other drugs.
- Klor-Con (potassium chloride) interacts with more than 100 other drugs.
- Oysco 500 with D (calcium/vitamin d) interacts with more than 200 other drugs.
Interactions between your selected drugs and food
morphine ↔ food
Major Drug Interaction
Applies to: Kadian (morphine)
GENERALLY AVOID: The central nervous system-depressant effects of morphine and alcohol may be additive. The combination may result in additive CNS-depression and impairment of judgment, thinking, and psychomotor skills. In more severe cases, respiratory depression, hypotension, profound sedation, and coma can occur.
GENERALLY AVOID: Consumption of alcohol while taking some sustained-release formulations of morphine may cause rapid release of the drug, resulting in high systemic levels of morphine that may be potentially lethal. Alcohol apparently can disrupt the release mechanism of some sustained-release formulations. The interaction was observed in in vitro studies using a 24-hour morphine formulation (Avinza 30 mg capsule, available in the U.S. from Ligand Pharmaceuticals). When the capsule was mixed with 900 mL of buffer solutions containing ethanol 20% and 40%, the dose of morphine that was released was alcohol concentration-dependent, leading to a more rapid release of morphine. Although the clinical relevance of this finding is unknown, 'dose-dumping' into the bloodstream is conceivable.
MANAGEMENT: Until more information is available, patients taking sustained-release formulations of morphine should not consume alcohol or use medications that contain alcohol. In general, potent narcotics such as morphine should not be combined with alcohol.
methotrexate ↔ food
Moderate Drug Interaction
Applies to: methotrexate
MONITOR: Limited data suggest that consumption of greater than 180 mg/day of caffeine may interfere with the efficacy of methotrexate (MTX) in patients with rheumatoid arthritis. The exact mechanism of interaction is unknown but may be related to the antagonistic effect of caffeine on adenosine receptors, as anti-inflammatory properties of MTX is thought to result from the accumulation of adenosine. In a study of 39 patients treated with MTX 7.5 mg/week (without folate supplementation) for 3 months, patients with high caffeine intake (more than 180 mg/day) experienced significantly less improvement in morning stiffness and joint pain from baseline than patients with low caffeine intake (more than 120 mg/day). There were no significant differences between the responses of patients with moderate caffeine intake (120 to 180 mg/day) and those of the other 2 groups. In an interview of 91 patients treated with MTX, 26% of patients who discontinued the drug were regular coffee drinkers compared to only 2% of those still receiving the drug. Because treatment failure was the reason for MTX discontinuation in 80% of patients who discontinued, the investigators suggested that caffeine may have interfered with MTX efficacy.
MANAGEMENT: Until further information is available, the potential for interaction should be considered in patients who consume substantial amounts of caffeine and caffeine-containing foods and are prescribed methotrexate for rheumatoid arthritis. It may be appropriate to limit caffeine intake if an interaction is suspected in cases of treatment failure.
diazepam ↔ food
Moderate Drug Interaction
Applies to: diazepam
MONITOR: Grapefruit juice may increase the plasma concentrations of some orally administered drugs that are primarily metabolized by the CYP450 3A4 isoenzyme. The proposed mechanism is inhibition of CYP450 3A4-mediated first-pass metabolism in the gut wall by certain compounds present in grapefruits. The extent and clinical significance of many of these interactions are unknown. Moreover, pharmacokinetic alterations associated with interactions involving grapefruit juice are often subject to a high degree of interpatient variability.
MANAGEMENT: Patients who regularly consume grapefruits and grapefruit juice should be monitored for adverse effects and altered plasma concentrations of drugs that are metabolized by CYP450 3A4. Grapefruits and grapefruit juice should be avoided if an interaction is suspected. Orange juice is not expected to interact with these drugs.
ergotamine ↔ food
Moderate Drug Interaction
Applies to: Cafergot (caffeine/ergotamine)
MONITOR: Grapefruit juice may increase the plasma concentrations of some orally administered drugs that are primarily metabolized by the CYP450 3A4 isoenzyme. The proposed mechanism is inhibition of CYP450 3A4-mediated first-pass metabolism in the gut wall by certain compounds present in grapefruits. The extent and clinical significance of many of these interactions are unknown. Moreover, pharmacokinetic alterations associated with interactions involving grapefruit juice are often subject to a high degree of interpatient variability.
MANAGEMENT: Patients who regularly consume grapefruits and grapefruit juice should be monitored for adverse effects and altered plasma concentrations of drugs that are metabolized by CYP450 3A4. Grapefruits and grapefruit juice should be avoided if an interaction is suspected. Orange juice is not expected to interact with these drugs.
levothyroxine ↔ food
Moderate Drug Interaction
Applies to: levothyroxine
ADJUST DOSING INTERVAL: Consumption of certain foods as well as the timing of meals relative to dosing may affect the absorption of T4 thyroid hormone (i.e., levothyroxine). T4 absorption is increased by fasting and decreased by foods such as soybean flour (e.g., infant formula), cotton seed meal, walnuts, dietary fiber, calcium, and calcium fortified juices.
MANAGEMENT: Preparations containing T4 thyroid hormone should be administered on a consistent schedule with regard to time of day and relation to meals so as to avoid large fluctuations in serum levels. Foods that may affect T4 absorption should be avoided within several hours of dosing if possible.
losartan ↔ food
Moderate Drug Interaction
Applies to: Cozaar (losartan)
GENERALLY AVOID: Moderate-to-high dietary intake of potassium, especially salt substitutes, may increase the risk of hyperkalemia in some patients who are using angiotensin II receptor blockers (ARBs). ARBs can promote hyperkalemia through inhibition of angiotensin II-induced aldosterone secretion. Patients with diabetes, heart failure, dehydration, or renal insufficiency have a greater risk of developing hyperkalemia.
MANAGEMENT: Patients should receive dietary counseling and be advised to not use potassium-containing salt substitutes or over-the-counter potassium supplements without consulting their physician. If salt substitutes are used concurrently, regular monitoring of serum potassium levels is recommended. Patients should also be advised to seek medical attention if they experience symptoms of hyperkalemia such as weakness, irregular heartbeat, confusion, tingling of the extremities, or feelings of heaviness in the legs.
MONITOR: Grapefruit juice may modestly decrease and delay the conversion of losartan to its active metabolite, E3174. The proposed mechanism is inhibition of CYP450 3A4-mediated first-pass metabolism in the gut wall by certain compounds present in grapefruits. The clinical significance is unknown. Moreover, pharmacokinetic alterations associated with interactions involving grapefruit juice are often subject to a high degree of interpatient variability.
MANAGEMENT: Patients who regularly consume grapefruits and grapefruit juice should be monitored for altered efficacy of losartan. Grapefruits and grapefruit juice should be avoided if an interaction is suspected. Orange juice is not expected to interact.
caffeine ↔ food
Minor Drug Interaction
Applies to: Ascomp with Codeine (aspirin/butalbital/caffeine/codeine), Cafergot (caffeine/ergotamine)
The effect of grapefruit juice on the pharmacologic activity of caffeine is controversial. One report suggests that grapefruit juice increases the effect of caffeine. The proposed mechanism is inhibition of cytochrome P-450 metabolism of caffeine. However, a well-conducted pharmacokinetic/pharmacodynamic study did not demonstrate this effect. The clinical significance of this potential interaction is unknown.
loratadine ↔ food
Minor Drug Interaction
Applies to: loratadine
Theoretically, grapefruit juice may increase the plasma concentrations of loratadine as it does other drugs that are substrates of the CYP450 3A4 enzymatic pathway. The proposed mechanism is inhibition of CYP450 3A4-mediated first-pass metabolism in the gut wall by certain compounds present in grapefruits. The clinical significance of this potential interaction is unknown. Reported interactions with potent CYP450 3A4 inhibitors like clarithromycin, erythromycin and ketoconazole have produced substantial increases in the area under the plasma concentration-time curve (AUC) of loratadine and its active metabolite, descarboethoxyloratadine, without associated changes in the overall safety profile of the drug.
______________________________________________
______________________________________________
MICARDIS
Micardis HCT
All medicines may cause side effects, but many people have no, or minor, side effects. Check with your doctor if any of these most COMMON side effects persist or become bothersome when using Micardis HCT:
Back pain; diarrhea; dizziness; nausea; tiredness.
Seek medical attention right away if any of these SEVERE side effects occur when using Micardis HCT:
Severe allergic reactions (rash; hives; itching; difficulty breathing; tightness in the chest; swelling of the mouth, face, lips, or tongue; unusual hoarseness); chest pain; confusion; decrease in sexual ability; decreased or painful urination; depression; drowsiness; fainting; fast or irregular heartbeat; fever, chills, or persistent sore throat; increased or excessive sweating; muscle pain, tenderness, or cramps; red, swollen, blistered, or peeling skin; restlessness; seizures; severe or persistent dry mouth; shortness of breath; swelling of the arms or legs; unusual bruising or bleeding; unusual thirst, tiredness, or weakness; vomiting; yellowing of the skin or eyes.
This is not a complete list of all side effects that may occur. If you have questions about side effects, contact your health care provider. Call your doctor for medical advice about side effects. To report side effects to the appropriate agency, please read the Guide to Reporting Problems to FDA.
Top
Micardis HCT Side Effects - for the Professional
Micardis HCT
Micardis® HCT (telmisartan and hydrochlorothiazide) tablets has been evaluated for safety in over 1700 patients, including 716 treated for over six months and 420 for over one year. In clinical trials with Micardis HCT tablets, no unexpected adverse events have been observed. Adverse experiences have been limited to those that have been previously reported with telmisartan and/or hydrochlorothiazide. The overall incidence of adverse experiences reported with the combination was comparable to placebo. Most adverse experiences were mild in intensity and transient in nature and did not require discontinuation of therapy.
Adverse events occurring at an incidence of 2% or more in patients treated with telmisartan/hydrochlorothiazide and at a greater rate than in patients treated with placebo, irrespective of their causal association, are presented in Table 1.
TABLE 1 Adverse Events Occurring in ≥ 2% of Telmisartan/Hydrochlorothiazide (HCTZ) Patients* Telm/HCTZ Placebo Telm HCTZ
(N=414) (N=74) (N=209) (N=121)
(%) (%) (%) (%)
* includes all doses of telmisartan (20-160 mg), hydrochlorothiazide (6.25-25 mg), and combinations thereof
Body as a whole
Fatigue 3 1 3 3
Influenza-like
symptoms 2 1 2 3
Central/peripheral nervous system
Dizziness 5 1 4 6
Gastrointestinal system
Diarrhea 3 0 5 2
Nausea 2 0 1 2
Respiratory system disorder
Sinusitis 4 3 3 6
Upper respiratory
tract infection 8 7 7 10
The following adverse events were reported at a rate less than 2% in patients treated with telmisartan/hydrochlorothiazide and at a greater rate than in patients treated with placebo: back pain, dyspepsia, vomiting, tachycardia, hypokalemia, bronchitis, pharyngitis, rash, hypotension postural, abdominal pain.
Finally, the following adverse events were reported at a rate of 2% or greater in patients treated with telmisartan/hydrochlorothiazide, but were as, or more common in the placebo group: pain, headache, cough, urinary tract infection.
Adverse events occurred at approximately the same rates in men and women, older and younger patients, and black and non-black patients.
In controlled trials (n=1017), 0.3% of patients treated with Micardis® HCT (telmisartan and hydrochlorothiazide) tablets 40/12.5 mg, 80/12.5 mg or 80/25 mg discontinued due to orthostatic hypotension, and the incidence of dizziness was 4%, 7%, and 1% respectively.
Telmisartan
Other adverse experiences that have been reported with telmisartan, without regard to causality, are listed below:
Autonomic Nervous System: impotence, increased sweating, flushing
Body as a Whole: allergy, fever, leg pain, malaise, chest pain
Cardiovascular: palpitation, dependent edema, angina pectoris, leg edema, abnormal ECG, hypertension, peripheral edema
CNS: insomnia, somnolence, migraine, vertigo, paresthesia, involuntary muscle contractions, hypoaesthesia
Gastrointestinal: flatulence, constipation, gastritis, dry mouth, hemorrhoids, gastroenteritis, enteritis, gastroesophageal reflux, toothache, non-specific gastrointestinal disorders
Metabolic: gout, hypercholesterolemia, diabetes mellitus
Musculoskeletal: arthritis, arthralgia, leg cramps, myalgia
Psychiatric: anxiety, depression, nervousness
Resistance Mechanism: infection, fungal infection, abscess, otitis media
Respiratory: asthma, rhinitis, dyspnea, epistaxis
Skin: dermatitis, eczema, pruritus
Urinary: micturition frequency, cystitis
Vascular: cerebrovascular disorder
Special Senses: abnormal vision, conjunctivitis, tinnitus, earache
A single case of angioedema was reported (among a total of 3781 patients treated with telmisartan).
Hydrochlorothiazide
Other adverse experiences that have been reported with hydrochlorothiazide, without regard to causality, are listed below:
Body as a whole: weakness
Digestive: pancreatitis, jaundice (intrahepatic cholestatic jaundice), sialadenitis, cramping, gastric irritation
Hematologic: aplastic anemia, agranulocytosis, leukopenia, hemolytic anemia, thrombocytopenia
Hypersensitivity: purpura, photosensitivity, urticaria, necrotizing angiitis (vasculitis and cutaneous vasculitis), fever, respiratory distress including pneumonitis and pulmonary edema, anaphylactic reactions
Metabolic: hyperglycemia, glycosuria, hyperuricemia
Musculoskeletal: muscle spasm
Nervous System/Psychiatric: restlessness
Renal: renal failure, renal dysfunction, interstitial nephritis
Skin: erythema multiforme including Stevens-Johnson syndrome, exfoliative dermatitis including toxic epidermal necrolysis
Special Senses: transient blurred vision, xanthopsia
Post-Marketing Experience
The following adverse reactions have been identified during post-approval use of Micardis® (telmisartan) tablets. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate reliably their frequency or establish a causal relationship to drug exposure. Decisions to include these reactions in labeling are typically based on one or more of the following factors: (1) seriousness of the reaction, (2) frequency of reporting, or (3) strength of causal connection to MICARDIS tablets. The most frequently spontaneously reported events include: headache, dizziness, asthenia, coughing, nausea, fatigue, weakness, edema, face edema, lower limb edema, angioneurotic edema, urticaria, hypersensitivity, sweating increased, erythema, chest pain, atrial fibrillation, congestive heart failure, myocardial infarction, blood pressure increased, hypertension aggravated, hypotension (including postural hypotension), hyperkalemia, syncope, dyspepsia, diarrhea, pain, urinary tract infection, erectile dysfunction, back pain, abdominal pain, muscle cramps (including leg cramps), myalgia, bradycardia, eosinophilia, thrombocytopenia, uric acid increased, abnormal hepatic function/liver disorder (more commonly seen in Japanese patients), renal impairment including acute renal failure, anemia, increased CPK, anaphylactic reaction, tendon pain (including tendonitis, tenosynovitis), drug eruption (toxic skin eruption mostly reported as toxicoderma, rash, and urticaria), hypoglycemia (in diabetic patients), and angioedema (with fatal outcome).
Rare cases of rhabdomyolysis have been reported in patients receiving angiotensin II receptor blockers, including MICARDIS tablets.
Clinical Laboratory Findings
In controlled trials, clinically relevant changes in standard laboratory test parameters were rarely associated with administration of Micardis® HCT (telmisartan and hydrochlorothiazide) tablets.
Hemoglobin and Hematocrit: Decreases in hemoglobin (≥ 2 g/dL) and hematocrit (≥ 9%) were observed in 1.2% and 0.6% of telmisartan/hydrochlorothiazide patients, respectively, in controlled trials. Changes in hemoglobin and hematocrit were not considered clinically significant and there were no discontinuations due to anemia.
Creatinine, Blood Urea Nitrogen (BUN): Increases in BUN (≥ 11.2 mg/dL) and serum creatinine (≥ 0.5 mg/dL) were observed in 2.8% and 1.4%, respectively, of patients with essential hypertension treated with Micardis HCT tablets in controlled trials. No patient discontinued treatment with Micardis HCT tablets due to an increase in BUN or creatinine.
Liver Function Tests: Occasional elevations of liver enzymes and/or serum bilirubin have occurred. No telmisartan/hydrochlorothiazide treated patients discontinued therapy due to abnormal hepatic function.
Serum Electrolytes: See PRECAUTIONS.
Top
Ads by Google
Treatment For Depression
Topicologist.comFind The Best Depression Treatment & How It Can Help Manage Symptoms
Begin Drug Treatment
www.TheTreatmentCenter.comChange Your Life In A Safe Retreat. Call Us 24/7. Insurance Welcome.
chemo side effects
www.MyTreatmentDecision.comLearn about a test that can help determine your benefit from chemo
Side Effects by Body System - for Healthcare Professionals
General
In general, prior to approval by the FDA, hydrochlorothiazide-telmisartan was evaluated for safety in more than 1,700 patients, including over 700 treated for over 6 months, and 420 for over 1 year. Adverse experiences have generally been mild and transient in nature and have only infrequently required discontinuation of therapy. The overall incidence of adverse experiences associated with the use of hydrochlorothiazide-telmisartan was comparable to placebo or to monotherapy with telmisartan or hydrochlorothiazide. The overall frequency of adverse experiences was not related to gender, age, or race.
Cardiovascular
Cardiovascular side effects associated with hydrochlorothiazide-telmisartan including tachycardia and orthostatic hypotension have been reported in less than 2% of patients. Cardiac arrhythmias, ventricular ectopy, and complete AV heart block that are associated with hypokalemia and hyponatremia have been reported with the use of HCTZ. Hypotension has been reported in association with HCTZ-induced pulmonary edema.
Cardiovascular side effects associated with the use of angiotensin II receptor inhibitors have included dizziness (related to orthostatic hypotension), palpitations, and chest pain. Cardiovascular side effects for telmisartan in relation to comparator therapy have included peripheral edema (1.0% vs. 0.0%). Chest pain, atrial fibrillation, congestive heart failure, myocardial infarction, increased blood pressure, aggravated hypertension, hypotension, and postural hypotension have been reported with the use of telmisartan in postmarketing experience.
In controlled clinical trials, discontinuation of therapy due to orthostatic hypotension occurred in 0.2% of treated patients.
Orthostatic hypotension may occur and may rarely be associated with syncope, particularly in the elderly.
Dermatologic
Dermatologic side effects associated with HCTZ have included case reports of erythema annulare centrifugum and acute eczematous dermatitis. Thiazides may induce phototoxic dermatitis. In addition, a rare, distinct entity with clinical and laboratory features indistinguishable from those of subacute cutaneous lupus erythematosus has been reported with the use of HCTZ. Rash has been reported in less than 1% of patients.
Dermatologic side effects associated with telmisartan including pruritus, rash, eczema, and general dermatitis have been reported rarely and at rates similar to placebo.
A 67-year-old woman with hypothyroidism, hypercalcemia, depression, and hypertension developed facial erythema, headaches, tremors, confusion and personality changes associated with a new positive ANA and anti-nRNP, and a skin biopsy consistent with lupus erythematosus while taking HCTZ, levothyroxine, and amitriptyline. The eruption resolved upon discontinuation of HCTZ, but she later developed a higher ANA titer associated with symptomatic diffuse interstitial pulmonary infiltrates. She was successfully treated with corticosteroids.
Endocrine
Endocrinologic side effects associated with thiazide diuretics have been reported rarely. A single case of recurrent parathyroid adenoma is reported, although the association is probably coincidental.
Gastrointestinal
Thiazide diuretics may increase serum cholesterol and triglycerides, resulting in increased risk of cholesterol gallstone formation. Reports of bowel strictures associated with thiazide ingestion have been reported, although these patients were on a combination HCTZ-potassium product.
Gastrointestinal side effects associated with HCTZ have been reported rarely. These have included case reports of pancreatitis and acute cholecystitis. Nausea, vomiting, and diarrhea have been reported in less than 1% of patients. Bowel strictures have been reported.
Gastrointestinal effects associated with telmisartan have including diarrhea (3%), flatulence, abdominal pain, nausea, constipation, gastritis, vomiting, dry mouth, dyspepsia, hemorrhoids, gastroenteritis, enteritis, and gastroesophageal reflux have been reported in less than 1% of patients and at rates similar to placebo. A case of acute pancreatitis has been reported. Cholecystitis and cholelithiasis have been reported.
Genitourinary
Genitourinary side effects associated with telmisartan have been reported extremely rarely and at rates similar to placebo. These have included impotence, urinary frequency, and cystitis. Interstitial cystitis has been reported rarely. Urinary tract infection and erectile dysfunction have been reported during postmarketing experience. Endometriosis has been reported.
Hematologic
Hematologic side effects associated with HCTZ have been reported rarely. Cases of immune-complex hemolytic anemia, aplastic anemia, and thrombocytopenia have been reported.
Hematologic side effects associated with telmisartan have been reported rarely. Decreased hemoglobin (greater than 2 g/dL) has been reported in 0.8% of patients compared to 0.3% in placebo. Granuloma has been reported.
Hepatic
Hepatic side effects associated with telmisartan including occasional elevations of serum hepatic enzymes have been reported rarely and in some studies at rates slightly greater than placebo.
Hypersensitivity
There have been approximately 34 known cases of thiazide-induced pulmonary edema, encompassing 52 episodes of pulmonary edema. In some cases, doses as small as 12.5 mg were associated with the development of pulmonary edema. The average time to onset of this adverse reaction is 44 minutes, women carry a relative risk of 9 to 1, and the average age is 56 years. The mortality rate is 6%. Some experts consider this side effect grossly underreported. These cases of acute noncardiogenic pulmonary edema are thought to be due to idiosyncrasy or a hypersensitivity mechanism.
Hypersensitivity side effects associated with HCTZ have been reported rarely. Cases of acute pulmonary edema and anaphylaxis have been reported. These have included approximately 30 case reports of acute noncardiogenic pulmonary edema. Morbilliform and leukocytoclastic vasculitis have been reported.
Hypersensitivity side effects associated with telmisartan including edema, face edema, lower limb edema, angioneurotic edema, hypersensitivity, erythema, angioedema, and urticaria have been reported during postmarketing experience. Angioedema has been reported with the use of angiotensin II receptor inhibitors.
Immunologic
Immunologic side effects associated with HCTZ have included numerous case reports of patients developing a rash histologically identical to subacute cutaneous lupus following HCTZ administration.
A 53-year-old man with hypertension developed nausea, vomiting, diarrhea, and progressive anorexia and weakness associated with scleral icterus, anemia with spherocytosis, dark red urine with proteinuria, bilirubinuria, hemoglobinuria, and elevated lactic dehydrogenase levels 18 months after beginning hydrochlorothiazide and methyldopa. Haptoglobin was less than 50 mg per dl. Direct and indirect Coombs tests were positive. The patient died suddenly; autopsy revealed no obvious cause of death, left ventricular hypertrophy, and mild coronary atherosclerosis.
Metabolic
Metabolic side effects associated with HCTZ have been reported frequently, especially when doses greater than 50 mg per day are used. These have included glucose intolerance (3%), metabolic alkalosis, hyponatremia, hypomagnesemia, hypercalcemia, hyperglycemia, and elevated serum uric acid levels. Mild hypokalemia (decrease of 0.5 mEq/L) have been reported in up to 50% of patients, and may predispose patients to cardiac arrhythmias. Increased (by 11%) total serum cholesterol, increased (by 12%) LDL lipoprotein cholesterol, and increased (by 50%) VLDL lipoprotein cholesterol have been reported.
Metabolic side effects associated with the use of telmisartan including gout, hypercholesterolemia, and hyperglycemia have been reported in less than 1% of patients. Causal relationships have not been shown, and these abnormalities were also noted among placebo patients. Hyperkalemia has been reported during postmarketing experience.
Musculoskeletal
Musculoskeletal side effects associated with hydrochlorothiazide have rarely included case reports of myalgias.
Musculoskeletal side effects associated with telmisartan including back pain has been reported in 3% of patients and at rates similar to placebo. Arthritis, arthralgias, myalgias, and leg pain have been reported in less than 1% of patients and at rates similar to placebo. Rhabdomyolysis has been reported during postmarketing experience in patients receiving angiotensin II receptor blockers including telmisartan. Muscle cramps (including leg cramps), and tendon pain (including tendonitis, tenosynovitis), myalgia, peripheral ischemia, and elevated creatine phosphokinase (CPK) have also been reported during postmarketing experience.
Nervous system
Although extremely rare cases of stroke have occurred during therapy with telmisartan, there is an expected incidence of stroke among all patients with hypertension.
Nervous system side effects including cerebrovascular insufficiency have been associated with HCTZ-induced plasma volume contraction.
Nervous system side effects associated with telmisartan including headache have been reported in 1% to 3.5% of patients.
Dizziness has been reported in up to 3.5% of patients. Pain, insomnia, somnolence, migraine, vertigo, tinnitus, paresthesias, involuntary muscle contractions, and hypoesthesias have been reported in less than 1% of patients and at rates similar to placebo. Stroke has been reported rarely. Asthenia, weakness, and syncope have been reported during postmarketing experience.
Ocular
Ocular side effects associated with telmisartan have rarely included abnormal vision and conjunctivitis.
Psychiatric
Psychiatric side effects associated with telmisartan have rarely included anxiety, depression, and nervousness.
Renal
Although hydrochlorothiazide has been used to treat nephrogenic diabetes insipidus, a case report in which the drug was believed to have caused this condition has been reported.
In some patients whose renal function is dependent upon the renin-angiotensin-aldosterone (RAA) system, such as patients with severe congestive heart failure, use of angiotensin II receptor inhibitors has been associated with oliguria and/or progressive azotemia and (rarely) with acute renal failure and/or death.
As with ACE inhibitors, use of angiotensin II receptor inhibitors can lead to increases in BUN and serum creatinine in patients with unilateral or bilateral renal artery stenosis.
Renal side effects including renal insufficiency manifesting as an increased serum creatinine and BUN have been reported due to HCTZ-induced intravascular volume depletion. Rare cases of interstitial nephritis have been reported and at a rate similar to placebo. A case of nephrogenic diabetes insipidus has been reported.
Renal side effects associated with telmisartan have been reported rarely. Increased (0.5 mg/dL or greater) serum creatinine (0.4% vs. 0.3% in placebo) and increased BUN has been reported. Oliguria and/or progressive azotemia have been reported with the use of angiotensin II receptor inhibitors. Rarely, acute renal failure and/or death have been reported.
Respiratory
Angiotensin II receptor blockade, unlike ACE inhibition, has no impact on the processing of peptides such as bradykinin and substance P, two peptides associated with the induction of cough.
Respiratory side effects associated with telmisartan including upper respiratory infections, sinusitis, and pharyngitis have been reported in 1% to 7% of patients and at rates similar to placebo. Bronchitis has been reported. Cough has been reported in 1.6% of patients at rates similar to placebo during postmarketing experience. Acute sinusitis has been reported.
Other
Other side effects associated with hydrochlorothiazide-telmisartan have included flu-like symptoms. Earaches and fatigue has been reported in less than 1% of patients and at rates similar to placebo. Chills have been reported rarely.
Other side effects for telmisartan in relation to comparator therapy have included fatigue (3.5% vs. 8.6%).
FYI:
online price
Telmisartan/HCTZ Generic 40/12.5mg 100 tablets $49.00
______________________________________________
______________________________________________
______________________________________________
New York City Poison Control Center, 455 1st Avenue, Room 123, New York, NY, 10016, U.S.A.
Department of Emergency Medicine, New York City Poison Control Center, NYU/Bellevue Hospital Center, New York, New York, U.S.A.
Department of Emergency Medicine, Central New York Regional Poison Control Center, Syracuse, New York, U.S.A.
Department of Critical Care, St. Anthony's Medical Center, St. Louis, Missouri, U.S.A.
Background: Although ingestion of sustained-release potassium supplements can cause life-threatening hyperkalemia in patients with abnormal renal function, only a few previous reports suggest that this may occur in patients with normal renal function. We report 2 cases of hyperkalemia in patients with normal renal function who developed hyperkalemia after ingesting sustained-release potassium preparations and describe the use of radiography and whole-bowel irrigation in their care. Case Reports: The first patient is a 50-year-old woman who ingested 100 K-Dur® tablets (each tablet containing 750 mg KCl or 10 mEq potassium) in a suicide attempt 1 hour prior to presenting to the emergency department. She developed a peak serum potassium level of 9.7 mEq/L and had transient, potentially life-threatening electrocardiographic changes. The second patient was a 17-year-old man who ingested 20 to 30 Klor-Con® tablets (each tablet containing 750 mg KCl or 10 mEq potassium) in a suicide attempt 10 hours prior to presentation. Although he developed a peak serum potassium level of 6.1 mEq/L, he had a persistently normal electrocardiogram. In both patients, the tablets were visualized on abdominal radiographs and the gastrointestinal tracts of both were successfully decontaminated using whole-bowel irrigation. Discussion: Although the sensitivity and specificity are unknown, the abdominal radiograph appears to be useful in detecting sustained-release potassium tablets. Whole-bowel irrigation as a primary method of gastrointestinal decontamination also appears to be effective although its use is not previously reported for sustained-release potassium overdoses.
Read More: http://informahealthcare.com/doi/abs/10.1081/CLT-100108499
______________________________________________
Among all the shit above, I have to look these up-
http://en.wikipedia.org/wiki/Osteomalacia
Osteomalacia
Clinical features
Osteomalacia in adults starts insidiously as aches and pains in the lumbar (lower back) region and thighs, spreading later to the arms and ribs. The pain is symmetrical, non-radiating and is accompanied by sensitivity in the involved bones. Proximal muscles are weak, and there is difficulty in climbing up stairs and getting up from a squatting position.
Due to demineralization bones become less rigid. Physical signs include deformities like triradiate pelvis[5] and lordosis. The patient has a typical "waddling" gait. However, those physical signs may derive from a previous osteomalacial state, since bones do not regain their original shape after they become deformed.
Pathologic fractures due to weight bearing may develop. Most of the time, the only alleged symptom is chronic fatigue, while bone aches are not spontaneous but only revealed by pressure or shocks.
It differs from renal osteodystrophy, where the latter shows hyperphosphatemia
______________________________________________
______________________________________________
Hyperkalemia
{This is the absolute scariest for me}.| Hyperkalemia | |
|---|---|
| Classification and external resources | |
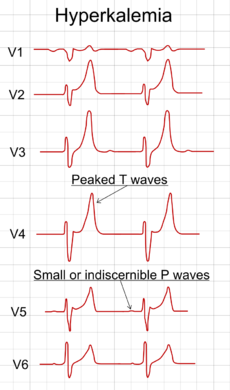 Electrocardiography showing precordial leads in hyperkalemia. | |
| ICD-10 | E87.5 |
| ICD-9 | 276.7 |
| DiseasesDB | 6242 |
| MedlinePlus | 001179 |
| eMedicine | emerg/261 |
| MeSH | D006947 |
Hyperkalemia (hyperkalaemia in British English, hyper- high; kalium, potassium; -emia, "in the blood") refers to the condition in which the concentration of the electrolyte potassium (K+) in the blood is elevated. Extreme hyperkalemia is a medical emergency due to the risk of potentially fatal abnormal heart rhythms (arrhythmia). The prefix hyper- means high (contrast with hypo-, meaning low). Kal refers to kalium, the Neo-Latin for potassium, and -emia means "in the blood."
Normal serum potassium levels are between 3.5 and 5.0 mEq/L;[1] at least 95% of the body's potassium is found inside cells, with the remainder in the blood. This concentration gradient is maintained principally by the Na+/K+ pump.
Contents |
Signs and symptoms
Symptoms are fairly nonspecific and generally include malaise, palpitations and muscle weakness; mild hyperventilation may indicate a compensatory response to metabolic acidosis, which is one of the possible causes of hyperkalemia. Often, however, the problem is detected during screening blood tests for a medical disorder, or it only comes to medical attention after complications have developed, such as cardiac arrhythmia or sudden death.
During the medical history taking, a physician will focus on kidney disease and medication use (see below), as these are the main causes. The combination of abdominal pain, hypoglycemia and hyperpigmentation, often in the context of a history of other autoimmune disorders, may be signs of Addison's disease, itself a medical emergency.
Causes
Ineffective elimination
- Renal insufficiency
- Medication that interferes with urinary excretion:
- ACE inhibitors and angiotensin receptor blockers
- Potassium-sparing diuretics (e.g. amiloride and spironolactone)
- NSAIDs such as ibuprofen, naproxen, or celecoxib
- The calcineurin inhibitor immunosuppressants ciclosporin and tacrolimus
- The antibiotic trimethoprim
- The antiparasitic drug pentamidine
- Mineralocorticoid deficiency or resistance, such as:
- Addison's disease
- Aldosterone deficiency
- Some forms of congenital adrenal hyperplasia
- Type IV renal tubular acidosis (resistance of renal tubules to aldosterone)
- Gordon's syndrome (“familial hypertension with hyperkalemia”), a rare genetic disorder caused by defective modulators of salt transporters, including the thiazide-sensitive Na-Cl cotransporter.
Excessive release from cells
- Rhabdomyolysis, burns or any cause of rapid tissue necrosis, including tumor lysis syndrome
- Massive blood transfusion or massive hemolysis
- Shifts/transport out of cells caused by acidosis, low insulin levels, beta-blocker therapy, digoxin overdose, or the paralyzing agent succinylcholine
Excessive intake
- Excess intake with salt-substitute, potassium-containing dietary supplements, or potassium chloride (KCl) infusion. Note that for a person with normal kidney function and nothing interfering with normal elimination (see above), hyperkalemia by potassium intake would be seen only with large infusions of KCl or oral doses of several hundred milliequivalents of KCl.[2]
Lethal injection
Wait... wtf? ?!?!?!?!
In the United States of America, hyperkalemia is intentionally brought about in an execution by lethal injection. A lethal dose of potassium chloride is the third and last of the three drugs administered, and the one that actually causes death.
Pseudohyperkalemia
Pseudohyperkalemia is a rise in the amount of potassium that occurs due to excessive leakage of potassium from cells, during or after blood is drawn. It is a laboratory artifact rather than a biological abnormality and can be misleading to caregivers.[3] Pseudohyperkalemia is typically caused by hemolysis during venipuncture (by either excessive vacuum of the blood draw or by a collection needle that is of too fine a gauge); excessive tourniquet time or fist clenching during phlebotomy (which presumably leads to efflux of potassium from the muscle cells into the bloodstream);[4] or by a delay in the processing of the blood specimen. It can also occur in specimens from patients with abnormally high numbers of platelets (>500,000/mm³), leukocytes (> 70 000/mm³), or erythrocytes (hematocrit > 55%). People with "leakier" cell membranes have been found, whose blood must be separated immediately to avoid pseudohyperkalemia.[5]
Pathophysiology
Potassium is the most abundant intracellular cation. It is critically important for many physiological processes, including maintenance of cellular membrane potential, homeostasis of cell volume, and transmission of action potentials in nerve cells. Its main dietary sources are vegetables (tomato and potato), fruits (orange and banana) and meat. Elimination is through the gastrointestinal tract and the kidney.
The renal elimination of potassium is passive (through the glomeruli), and reabsorption is active in the proximal tubule and the ascending limb of the loop of Henle. There is active excretion of potassium in the distal tubule and the collecting duct; both are controlled by aldosterone.
Hyperkalemia develops when there is excessive production (oral intake, tissue breakdown) or ineffective elimination of potassium. Ineffective elimination can be hormonal (in aldosterone deficiency) or due to causes in the renal parenchyma that impair excretion.
Increased extracellular potassium levels result in depolarization of the membrane potentials of cells. This depolarization opens some voltage-gated sodium channels, but not enough to generate an action potential. After a short while, the open sodium channels inactivate and become refractory, increasing the threshold needed to generate an action potential. This leads to the impairment of neuromuscular, cardiac, and gastrointestinal organ systems. Of most concern is the impairment of cardiac conduction which can result in ventricular fibrillation or asystole.
During extreme exercise, potassium is released from active muscle and the serum potassium rises to a point that would be dangerous at rest. For unclear reasons, it appears as if the high levels of adrenaline and noradrenaline have a protective effect on the cardiac electrophysiology.[6]
Patients with the rare hereditary condition of hyperkalemic periodic paralysis appear to have a heightened sensitivity of muscular symptoms that are associated with transient elevation of potassium levels. Episodes of muscle weakness and spasms can be precipitated by exercise or fasting in these subjects.
Diagnosis
To gather enough information for diagnosis, the measurement of potassium needs to be repeated, as the elevation can be due to hemolysis in the first sample. The normal serum level of potassium is 3.5 to 5 mEq/L. Generally, blood tests for renal function (creatinine, blood urea nitrogen), glucose and occasionally creatine kinase and cortisol will be performed. Calculating the trans-tubular potassium gradient can sometimes help in distinguishing the cause of the hyperkalemia.
In many cases, renal ultrasound will be performed, since hyperkalemia is highly suggestive of renal failure.
Also, electrocardiography (EKG/ECG) may be performed to determine if there is a significant risk of cardiac arrhythmias.
ECG findings
With mild to moderate hyperkalemia, there is reduction of the size of the P wave and development of peaked T waves. Severe hyperkalemia results in a widening of the QRS complex, and the EKG complex can evolve to a sinusoidal shape. There appears to be a direct effect of elevated potassium on some of the potassium channels that increases their activity and speeds membrane repolarization. Also, (as noted above), hyperkalemia causes an overall membrane depolarization that inactivates many sodium channels. The faster repolarization of the cardiac action potential causes the tenting of the T waves, and the inactivation of sodium channels causes a sluggish conduction of the electrical wave around the heart, which leads to smaller P waves and widening of the QRS complex.
The serum K+ concentration at which electrocardiographic changes develop is somewhat variable.[7][8] Although the factors influencing the effect of serum potassium levels on cardiac electrophysiology are not entirely understood, the concentrations of other electrolytes, as well as levels of catecholamines, play a major role.[9][10]
Treatment
When arrhythmias occur, or when potassium levels exceed 6.5 mmol/l, emergency lowering of potassium levels is mandated. Several agents are used to transiently lower K+ levels. Choice depends on the degree and cause of the hyperkalemia, and other aspects of the patient's condition.
Myocardial excitability
Calcium (Calcium chloride or calcium gluconate) increases threshold potential through a mechanism that is still unclear, thus restoring normal gradient between threshold potential and resting membrane potential, which is elevated abnormally in hyperkalemia. One ampule of Calcium chloride has approximately 3 times more calcium than calcium gluconate. Onset of action is <5 min and lasts about 30-60 min. Doses should be titrated with constant monitoring of ECG changes during administration and the dose should be repeated if ECG changes do not normalize within 3 to 5 min.
Lowering K+ temporarily
Several medical treatments shift potassium ions from the bloodstream into the cellular compartment, thereby reducing the risk of complications. The effect of these measures tends to be short-lived, but may temporize the problem until potassium can be removed from the body.[11]
- Insulin (e.g. intravenous injection of 10-15 units of regular insulin along with 50ml of 50% dextrose to prevent hypoglycemia) will lead to a shift of potassium ions into cells, secondary to increased activity of the sodium-potassium ATPase. Its effects last a few hours, so it sometimes needs to be repeated while other measures are taken to suppress potassium levels more permanently.
- Bicarbonate therapy (e.g. 1 ampule (45mEq) infused over 5 minutes) is effective in cases of metabolic acidosis. The bicarbonate ion will stimulate an exchange of cellular H+ for Na+, thus leading to stimulation of the sodium-potassium ATPase.
- Salbutamol (albuterol, Ventolin) is a β2-selective catecholamine that is administered by nebulizer (e.g. 10–20 mg). This drug also lowers blood levels of K+ by promoting its movement into cells.
Increasing elimination
Severe cases require hemodialysis or hemofiltration, which are the most rapid methods of removing potassium from the body. These are typically used if the underlying cause cannot be corrected swiftly while temporizing measures are instituted or there is no response to these measures.
Polystyrene sulfonate with sorbitol (Kayexalate) either orally or rectally is widely used with the goal to lower potassium over several hours. Removal of potassium is assumed to require defecation. However, careful clinical trials to demonstrate the effectiveness of Kayexalate are lacking, and there are small risks of necrosis of the colon.[12]
Long-term prevention
Preventing recurrence of hyperkalemia typically involves reduction of dietary potassium, removal of an offending medication, and/or the addition of oral bicarbonate or a diuretic (such as furosemide or hydrochlorothiazide). Polystyrene sulfonate and sorbital (Kayexalate) is occasionally used on an ongoing basis to maintain lower serum levels of potassium. Concerns regarding its use are noted in the previous section.
References:
- ^ Kratz, A; Ferraro, M; Sluss, PM; Lewandrowski, KB; Ellender, Stacey M.; Peters, Christine C.; Kratz, Alexander; Ferraro, Maryjane et al. (2004). "Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Laboratory reference values". The New England journal of medicine 351 (15): 1548–63. doi:10.1056/NEJMcpc049016. PMID 15470219.
- ^ Su M, Stork C, Ravuri S, et al. (2001). "Sustained-release potassium chloride overdose". J. Toxicol. Clin. Toxicol. 39 (6): 641–8. doi:10.1081/CLT-100108499. PMID 11762675.
- ^ Sevastos, N; Theodossiades, G; Efstathiou, S; Papatheodoridis, GV; Manesis, E; Archimandritis, AJ (March 2006). "Pseudohyperkalemia in serum: the phenomenon and its clinical magnitude". J. Lab. Clin. Med. 147 (3): 139–144. doi:10.1016/j.lab.2005.11.008. PMID 16503244.
- ^ Don, BR; Sebastian, A; Cheitlin, M; Christiansen, M; Schambelan, M (May 1990). "Pseudohyperkalemia caused by fist clenching during phlebotomy". N. Engl. J. Med. 322 (18): 1290–1292. doi:10.1056/NEJM199005033221806. PMID 2325722.
- ^ Iolascon, A; Stewart, GW; Ajetunmobi, JF; et al. (May 1999). "Familial pseudohyperkalemia maps to the same locus as dehydrated hereditary stomatocytosis (hereditary xerocytosis)". Blood 93 (9): 3120–3123. PMID 10216110. http://www.bloodjournal.org/cgi/pmidlookup?view=long&pmid=10216110.
- ^ Lindinger, MI (April 1995). "Potassium regulation during exercise and recovery in humans: implications for skeletal and cardiac muscle". J. Mol. Cell. Cardiol. 27 (4): 1011–1022. doi:10.1016/0022-2828(95)90070-5. PMID 7563098. http://linkinghub.elsevier.com/retrieve/pii/0022-2828(95)90070-5.
- ^ Wrenn, KD; Slovis, CM; Slovis, BS (1991). "The ability of physicians to predict hyperkalemia from the ECG.". Annals of emergency medicine 20 (11): 1229–32. PMID 1952310.
- ^ Aslam, S; Friedman, EA; Ifudu, O (2002). "Electrocardiography is unreliable in detecting potentially lethal hyperkalaemia in haemodialysis patients.". Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 17 (9): 1639–42. PMID 12198216.
- ^ Surawicz, B (1967). "Relationship between electrocardiogram and electrolytes.". American heart journal 73 (6): 814–34. PMID 5338052.
- ^ Leitch, SP; Patterson, DJ (1994). "Interactive effects of K+, acidosis, and catecholamines on isolated rabbit heart: implications for exercise.". Journal of applied physiology (Bethesda, Md. : 1985) 77 (3): 1164–71. PMID 7836118.
- ^ Elliott MJ, Ronksley PE, Clase CM, Ahmed SB, Hemmelgarn BR (October 2010). "Management of patients with acute hyperkalemia". CMAJ 182 (15): 1631–5. doi:10.1503/cmaj.100461. PMC 2952010. PMID 20855477.
- ^ Sterns RH, Rojas M, Bernstein P, Chennupati S (May 2010). "Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective?". J. Am. Soc. Nephrol. 21 (5): 733–5. doi:10.1681/ASN.2010010079. PMID 20167700.
External links
- USDA National Nutrient Database for Standard Reference, Release 20]
| ||||||||||||||||||||||||||||||||
__________________________________
This potassium retention thing -(the hyperkalimemia) is just so bad- and I further wonder what part that wrong drug I was infused with played into the mix? Why would it have set anything off?
I can't even think anymore. Please help me?
I have so much pain all the time...
Got done with my shower, after banging my head on the wall in there- (Yes, I have a bathtub chair, but had to stand to rinse etc.
Suddenly, my back stung like a bee- about 3" worth right in the middle, and I couldn't move. I grabbed the shower rod, and swung and hit my forehead. At that point in time, the damned washer decided it was time to rinse and I got blasted with some freakin' hot water. I finally shut 'er down, and sat for a bit.
I had one of my canes in there- along with the grabber thingie (that doesn't work half the time), but crawled over the edge of the tub and got out.
Damn fine thing I turned the heat up before I got in the shower- or I'd have frozen by that time.
I sat on the outside of the bathtub chair, trying to put lotion on and comb my hair out, etc etc etc. That took an hour too-
I tried and tried- Took me damn near another hour just to be able to get my bra, undies and 3 socks on.
I finally called Casey. (She got here about 2:30).
Thank Chris5t I have at least one kid that talks to me.
And thank God she's a girl. hehehe
I have just 2 questions... 1.
1. WTF do I do now?
And... 2. Why am I still alive?
(Especially after learning and knowing all of this shit- and taking the pills that I was given by that doctor/man I trusted)?!??
The thing that REALLY gets me is that he looks into his pocket computer at the PDR- drugs indexes...
Can't he fucking read???? (Damn, now that's 3 questions).
XOXO
Me
For now i am only going to take the pain pills so i can at least move to get to the damn bathroom when the Diurex kicks in-
........ again and again and again and...
hehehe

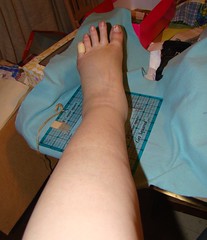
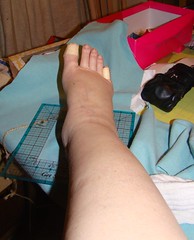
ReplyDeleteAny orange highlights up there are my boo-boos
They were supposed to be red.
XOXO
Me
ReplyDeleteSorry about the language too.
I am angry-
As if...
re: hypercalemia....
ReplyDeleteIn your Wikipedia article it says….
‘To gather enough information for diagnosis, the measurement of potassium needs to be repeated, as the elevation can be due to hemolysis in the first sample.’
Anne,
This happened to me… the first blood draw showed a high potassium level. Subsequent tests showed normal. What is your latest potassium level? Have you done a retest? It was the first thing my doctor did.
The edema you have is definitely WRONG. You need to see a doctor you can trust right away. Does your insurance allow a second opinion? How about Kelly’s doctor? Casey’s?
If you trust the folks at your pain clinic, call them for a dr. you can trust and get your butt in there.!
Jeesch! Please take care of yourself, I know you are trying..!
love,
k
Hi Sweet Mommy!
ReplyDeleteWOW! I can't believe all this stuff.... This is all so very incredibly scary for me and I can't even begin to fathom how you feel through all this...
My advice is to get in to see Dr. Szabo, my doctor... I know you don't want to go back to Oneida for anything, but maybe just get the edema fixed so that the stress on your heart goes down and you can be in less pain and less discomfort, and even more important: less worry. After that, I guess I'd recommend the same thing Ant Kris said and talk with Dr. Araujo or Becky Wolf at Pain Management and see who they can recommend.
This is way past a "simple mistake". Like I said to him about even the infusion mix-up (after he said, "No, it's my mistake") "That's a PRETTY BIG MISTAKE!!!"
I know what you mean about the PDR that he's got. They all have one, and many times Dr. Szabo has looked stuff up on there and if there's even the slightest chance of a bad reaction or a bad interaction with any of my other medications, he won't prescribe it.
Definitely contact a lawyer... The sooner the better. I'll help you to get pictures of all of the swelling because it's also in your arms/hands/wrists/etc. and the simple fact that he told you NOT TO WORRY about something as serious as this is WRONG WRONG WRONG! And worst of all, it's DANGEROUS!
I'm hoping that lawyer you will find will be able to bring all of this down on him and maybe even find more people whom he has treated this way (I know we heard from GB Home Medical that he fails to file papers or fails to help his patients get urgent and necessary medical equipment; and I know people in passing have said something about how awful he is). The more people engaged against him, the higher the chances are that he'll get stripped of his license to practice medicine.
I honestly think this man is malicious/sadistic. He never apologizes for any of these serious and potentially fatal errors, he tells you not to worry about severe interactions/side effects, tells you not to worry about the symptoms of a potentially fatal disease that you're showing that runs in your family... And the list goes on... He's never compassionate, the most I've ever heard him say is "hang in there"... Yet he never does anything to help get rid of the very problems his mistakes are creating!!! Like Greg said last night, "He's either malicious or just plain stupid, but either way he should NOT be practicing medicine." (Remember he was by my side when we went through all the crap this man pulled with my medical case and we know how severe that turned out to be)...
Okay, I think that's the advice I've got for you... Other than the obvious of discontinue the two meds involved in giving you the hyperkalemia, of course...
Remember, you have us all to back you up on this, and you're never alone! We'll get through this together, and kick this bastard where it counts... He can't be allowed to keep screwing around with people's lives; he will end up killing his patients if it hasn't happened in the past already... Call me if you need anything, I'm only a little ways away...
Know that I'm praying for you every day and night, and those prayers have got to be heard at that frequency, right? :) Know that someone(s) is (are) looking out for you on The Other Side, that you caught this and know all about what to watch out for now, and can discontinue stuff to prevent anything from further damaging your body... I SURELY THANK GOD AND EVERYONE ELSE PROTECTING YOU FOR THAT!!!!
Know also that I love you so very MUCH MUCH!! Forever and ever and ever!
~CaseyAnne
If you want a totally non-interested party you can go and ask my friend Mike. (Mike Unger on facebook). I have known him since college, he owns his own practice. I trust him as a doctor (just don't let him in your undies).... officially he is family medicine but he has also a good circle of his own trusted friends (many of whom I also know) who he might rather have you talk to.
ReplyDeleteHe can tell you who to contact within the medical world to get help and what steps to take. You should contact him directly as I know with patient confidentiality and all he won't like to go through a middle woman.
You need to file with the
Beyond that once you have an answer, call a lawyer. Any GOOD lawyer will take the case on contingency. Meaning, they don't get any money unless you do and they will not require any money up front.
No matter what you should absolutely talk to another doctor AND a lawyer. http://www.mcandl.com/wisconsin.html#I This link talks about your rights and responsibilities under Wisconsin law.
I would avoid going back to the same medical group as they would be more inclined to do less to protect themselves. They will NOT be impartial. I would go some place as non-related as possible.
You have as always, my thoughts and prayers tooo.